Oxaliplatin Eluting CalliSpheres Microspheres for the Treatment of Unresectable or Recurrent Hepatocellular Carcinoma
- PMID: 36034827
- PMCID: PMC9403481
- DOI: 10.3389/fphar.2022.923585
Oxaliplatin Eluting CalliSpheres Microspheres for the Treatment of Unresectable or Recurrent Hepatocellular Carcinoma
Abstract
Aim: Drug-eluting beads-transarterial chemoembolization (DEB-TACE) has been widely used in unresectable and advanced hepatocellular carcinoma (HCC). However, no study reported the clinical outcomes of drug-eluting beads TACE (DEB-TACE) with oxaliplatin-eluting CalliSpheres microspheres in the treatment of HCC. This study reports the preliminary outcomes of DEB-TACE loaded with oxaliplatin for the treatment of patients with unresectable or recurrent HCC. Methods: From November 2019 to November 2021, 29 patients with unresectable or recurrent HCC were recruited from our department and treated by DEB-TACE loaded with oxaliplatin. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were disease control rate and safety. Tumor response was investigated at 1, 3, and 6 months after DEB-TACE according to the criteria of the response evaluation in solid tumor (RECIST) criteria and the modified RECIST criteria (mRECIST). Survival curve was generated with the Kaplan-Meier method. Results: A total of 49 DEB-TACE sessions were performed, with a technical success rate of 100%. The overall response rate and disease control rate were 52.4 and 95.2%, 64.7 and 76.5%, and 54.5 and 63.3%, respectively, at 1, 3, and 6 months after DEB-TACE (mRECIST). The PFS was 5.9 months, and the median overall survival was 18.8 months. The 6- and 12-month overall survival rate was 82.5% and 67.5%, respectively, No treatment-related mortality or severe adverse events were observed. Minor complications were observed in 21 patients (72.4%), and abdominal pain (41.4%) was the most common treatment-related complication. Conclusion: DEB-TACE loaded with oxaliplatin-eluting CalliSpheres microspheres could be a safe, feasible, and efficacious palliative regimen in unresectable or recurrent HCC patients.
Keywords: CalliSpheres beads; TACE; drug-eluting beads transarterial chemoembolization (DEB-TACE); hepatocellular carcinoma (HCC); oxaliplatin.
Copyright © 2022 Bi, Ren, Ren, Ma and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
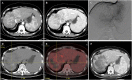
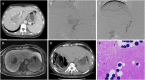
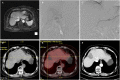
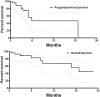
References
LinkOut - more resources
Full Text Sources
Miscellaneous

