Case Report: Clinical complete response of advanced renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion by treated by camrelizumab and axitinib: A rare case report
- PMID: 36034832
- PMCID: PMC9403306
- DOI: 10.3389/fphar.2022.927299
Case Report: Clinical complete response of advanced renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion by treated by camrelizumab and axitinib: A rare case report
Abstract
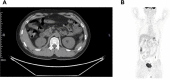
Renal cell carcinoma (RCC) associated with Xp11.2 translocation/TFE3 gene fusions is a rare subtype of renal tumor. This entity predominantly occurs in juveniles, but rarely in adults. Xp11.2 translocation RCC (tRCC) patients with lymph node or organ metastasis are associated with poor prognosis, and the strategy remains controversial. Herein, we presented our experience with the diagnosis and treatment of an adult case of Xp11.2 tRCC. In our clinical practice, a 32-year-old male manifested fever and right flank paroxysmal blunt pain, and computed tomography showed an inhomogeneous mass, 6 cm in diameter, in the right kidney. Then right partial nephrectomy (PN) and renal hilar lymph node dissection by laparoscopic surgery were performed. Pathology revealed that the tumor cells were positive for TFE3 immunohistologically and positive for TFE3 break-apart fluorescence in situ hybridization assay. A splice site mutation c.1544-1G>T of protein tyrosine phosphatase receptor delta (PTPRD) was detected by next-generation sequencing and weak PTPRD expression was confirmed in tumor tissues compared to tumor periphery. This patient was diagnosed with stage III RCC and received immune checkpoint inhibitor (camrelizumab) in combination with tyrosine kinase inhibitor (axitinib) treatment for 1 year. He achieved a clinical complete response with no sign of recurrence or metastasis. PTPRD mutation might be a favorable indicator for Xp11.2 tRCC patients managed by PN and followed by the adjuvant therapy of immune checkpoint inhibitor and tyrosine kinase inhibitor.
Keywords: PD-1; PTPRD-mutation; Xp11.2 translocation renal cell carcinoma; immunotherapy; tyrosine kinase inhibitor.
Copyright © 2022 Zhao, Dai, Xie, Fang, Chen, Dai and Xu.
Conflict of interest statement
Author KD and NC were employed by Hangzhou Jichenjunchuang Medical Laboratory Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Boilève A., Carlo M. I., Barthélémy P., Oudard S., Borchiellini D., Voss M. H., et al. (2018). Immune checkpoint inhibitors in MITF family translocation renal cell carcinomas and genetic correlates of exceptional responders. J. Immunother. Cancer 6 (1), 159. 10.1186/s40425-018-0482-z - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

