Chaperone activity of niflumic acid on ClC-1 chloride channel mutants causing myotonia congenita
- PMID: 36034862
- PMCID: PMC9403836
- DOI: 10.3389/fphar.2022.958196
Chaperone activity of niflumic acid on ClC-1 chloride channel mutants causing myotonia congenita
Abstract
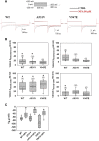
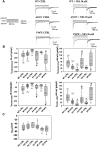
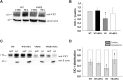
Myotonia congenita (MC) is an inherited rare disease characterized by impaired muscle relaxation after contraction, resulting in muscle stiffness. It is caused by loss-of-function mutations in the skeletal muscle chloride channel ClC-1, important for the stabilization of resting membrane potential and for the repolarization phase of action potentials. Thanks to in vitro functional studies, the molecular mechanisms by which ClC-1 mutations alter chloride ion influx into the cell have been in part clarified, classifying them in "gating-defective" or "expression-defective" mutations. To date, the treatment of MC is only palliative because no direct ClC-1 activator is available. An ideal drug should be one which is able to correct biophysical defects of ClC-1 in the case of gating-defective mutations or a drug capable to recover ClC-1 protein expression on the plasma membrane for trafficking-defective ones. In this study, we tested the ability of niflumic acid (NFA), a commercial nonsteroidal anti-inflammatory drug, to act as a pharmacological chaperone on trafficking-defective MC mutants (A531V, V947E). Wild-type (WT) or MC mutant ClC-1 channels were expressed in HEK293 cells and whole-cell chloride currents were recorded with the patch-clamp technique before and after NFA incubation. Membrane biotinylation assays and western blot were performed to support electrophysiological results. A531V and V947E mutations caused a decrease in chloride current density due to a reduction of ClC-1 total protein level and channel expression on the plasma membrane. The treatment of A531V and V947E-transfected cells with 50 µM NFA restored chloride currents, reaching levels similar to those of WT. Furthermore, no significant difference was observed in voltage dependence, suggesting that NFA increased protein membrane expression without altering the function of ClC-1. Indeed, biochemical experiments confirmed that V947E total protein expression and its plasma membrane distribution were recovered after NFA incubation, reaching protein levels similar to WT. Thus, the use of NFA as a pharmacological chaperone in trafficking defective ClC-1 channel mutations could represent a good strategy in the treatment of MC. Because of the favorable safety profile of this drug, our study may easily open the way for confirmatory human pilot studies aimed at verifying the antimyotonic activity of NFA in selected patients carrying specific ClC-1 channel mutations.
Keywords: ClC-1 chloride channel; drug repurposing; myotonia congenita; niflumic acid; precision medicine.
Copyright © 2022 Altamura, Conte, Campanale, Laghetti, Saltarella, Camerino, Imbrici and Desaphy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Altamura C., Lucchiari S., Sahbani D., Ulzi G., Comi G. P., D’Ambrosio P., et al. (2018a). The analysis of myotonia congenita mutations discloses functional clusters of amino acids within the CBS2 domain and the C-terminal peptide of the ClC-1 channel. Hum. Mutat. 39, 1273–1283. 10.1002/humu.23581 - DOI - PubMed
-
- Altamura C., Mangiatordi G. F., Nicolotti O., Sahbani D., Farinato A., Leonetti F., et al. (2018b). Mapping ligand binding pockets in chloride ClC-1 channels through an integrated in silico and experimental approach using anthracene-9-carboxylic acid and niflumic acid. Br. J. Pharmacol. 175 (10), 1770–1780. 10.1111/bph.14192 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

