Synthetic short-chain peptide analogues of H1 relaxin lack affinity for the RXFP1 receptor and relaxin-like bioactivity. Clues to a better understanding of relaxin agonist design
- PMID: 36034864
- PMCID: PMC9402926
- DOI: 10.3389/fphar.2022.942178
Synthetic short-chain peptide analogues of H1 relaxin lack affinity for the RXFP1 receptor and relaxin-like bioactivity. Clues to a better understanding of relaxin agonist design
Abstract
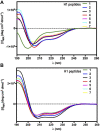
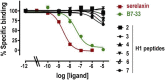
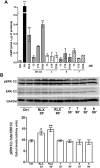
The peptide hormone relaxin (RLX), also available as clinical-grade recombinant protein (serelaxin), holds great promise as a cardiovascular and anti-fibrotic agent but is limited by the pharmacokinetic issues common to all peptide drugs. In this study, by a computational modelling chemistry approach, we have synthesized and tested a set of low molecular weight peptides based on the putative receptor-binding domain of the B chain of human H1 RLX isoform, with the objective to obtain RLX analogues with improved pharmacokinetic features. Some of them were stabilized to induce the appropriate 3-D conformation by intra-chain tri-azolic staples, which should theoretically enhance their resistance to digestive enzymes making them suited for oral administration. Despite these favourable premises, none of these H1 peptides, either linear or stapled, revealed a sufficient affinity to the specific RLX receptor RXFP1. Moreover, none of them was endowed with any RLX-like biological effects in RXFP1-expressing THP-1 human monocytic cells and mouse NIH-3T3-derived myofibroblasts in in vitro culture, in terms of significantly relevant cAMP elevation and ERK1/2 phosphorylation, which represent two major signal transduction events downstream RXFP1 activation. This was at variance with authentic serelaxin, which induced a clear-cut, significant activation of both these classical RLX signaling pathways. Albeit negative, the results of this study offer additional information about the structural requirements that new peptide therapeutics shall possess to effectively behave as RXFP1 agonists and RLX analogues.
Keywords: ERK1/2; RXFP1; RXFP1 agonists; cAMP; relaxin; relaxin analogues.
Copyright © 2022 D'Ercole, Nistri, Pacini, Carotenuto, Santoro, Papini, Bathgate, Bani and Rovero.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

