Genetic association-based functional analysis detects HOGA1 as a potential gene involved in fat accumulation
- PMID: 36035184
- PMCID: PMC9412052
- DOI: 10.3389/fgene.2022.951025
Genetic association-based functional analysis detects HOGA1 as a potential gene involved in fat accumulation
Abstract
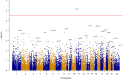
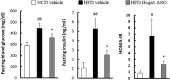
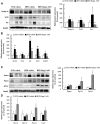
Although there are a number of discoveries from genome-wide association studies (GWAS) for obesity, it has not been successful in linking GWAS results to biology. We sought to discover causal genes for obesity by conducting functional studies on genes detected from genetic association analysis. Gene-based association analysis of 917 individual exome sequences showed that HOGA1 attains exome-wide significance (p-value < 2.7 × 10-6) for body mass index (BMI). The mRNA expression of HOGA1 is significantly increased in human adipose tissues from obese individuals in the Genotype-Tissue Expression (GTEx) dataset, which supports the genetic association of HOGA1 with BMI. Functional analyses employing cell- and animal model-based approaches were performed to gain insights into the functional relevance of Hoga1 in obesity. Adipogenesis was retarded when Hoga1 was knocked down by siRNA treatment in a mouse 3T3-L1 cell line and a similar inhibitory effect was confirmed in mice with down-regulated Hoga1. Hoga1 antisense oligonucleotide (ASO) treatment reduced body weight, blood lipid level, blood glucose, and adipocyte size in high-fat diet-induced mice. In addition, several lipogenic genes including Srebf1, Scd1, Lp1, and Acaca were down-regulated, while lipolytic genes Cpt1l, Ppara, and Ucp1 were up-regulated. Taken together, HOGA1 is a potential causal gene for obesity as it plays a role in excess body fat development.
Keywords: 3T3-L1; HOGA1; adipogenesis; association analysis; exome sequencing; functional study; obesity.
Copyright © 2022 Kim, Park, Ahn, Lim, Kwak, Randy, Song, Park, Nho and Cho.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous

