Identification of a cytokine profile in serum and cerebrospinal fluid of pediatric and adult spinal muscular atrophy patients and its modulation upon nusinersen treatment
- PMID: 36035258
- PMCID: PMC9406526
- DOI: 10.3389/fncel.2022.982760
Identification of a cytokine profile in serum and cerebrospinal fluid of pediatric and adult spinal muscular atrophy patients and its modulation upon nusinersen treatment
Abstract
Background and objectives: Multisystem involvement in spinal muscular atrophy (SMA) is gaining prominence since different therapeutic options are emerging, making the way for new SMA phenotypes and consequent challenges in clinical care. Defective immune organs have been found in preclinical models of SMA, suggesting an involvement of the immune system in the disease. However, the immune state in SMA patients has not been investigated so far. Here, we aimed to evaluate the innate and adaptive immunity pattern in SMA type 1 to type 3 patients, before and after nusinersen treatment.
Methods: Twenty one pediatric SMA type 1, 2, and 3 patients and 12 adult SMA type 2 and 3 patients were included in this single-center retrospective study. A Bio-Plex Pro-Human Cytokine 13-plex Immunoassay was used to measure cytokines in serum and cerebrospinal fluid (CSF) of the study cohort before and after 6 months of therapy with nusinersen.
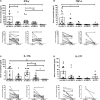
Results: We detected a significant increase in IL-1β, IL-4, IL-6, IL-10, IFN-γ, IL-17A, IL-22, IL-23, IL-31, and IL-33, in serum of pediatric and adult SMA patients at baseline, compared to pediatric reference ranges and to adult healthy controls. Pediatric patients showed also a significant increase in TNF-α and IL-17F levels at baseline. IL-4, IFN-γ, Il-22, IL-23, and IL-33 decreased in serum of pediatric SMA patients after 6 months of therapy when compared to baseline. A significant decrease in IL-4, IL-6, INF-γ, and IL-17A was detected in serum of adult SMA patients after treatment. CSF of both pediatric and adult SMA patients displayed detectable levels of all cytokines with no significant differences after 6 months of treatment with nusinersen. Notably, a higher baseline expression of IL-23 in serum correlated with a worse motor function outcome after treatment in pediatric patients. Moreover, after 6 months of treatment, patients presenting a higher IL-10 concentration in serum showed a better Hammersmith Functional Motor Scale Expanded (HFMSE) score.
Discussion: Pediatric and adult SMA patients show an inflammatory signature in serum that is reduced upon SMN2 modulating treatment, and the presence of inflammatory mediators in CSF. Our findings enhance SMA knowledge with potential clinical and therapeutic implications.
Keywords: SMA; biomarker; immune system; multisystemic; nusinersen.
Copyright © 2022 Bonanno, Cavalcante, Salvi, Giagnorio, Malacarne, Cattaneo, Andreetta, Venerando, Pensato, Gellera, Zanin, Arnoldi, Dosi, Mantegazza, Masson, Maggi and Marcuzzo.
Conflict of interest statement
SB received honoraria for advisory board activities, and compensation for travel and congress participation from Sanofi Genzyme, Biogen and Roche. RZ received funds for travel, congress participation and advisory board activities from Biogen. CD received consultancy fees from Novartis Gene Therapies. RiM was PI of SMA clinical trials for Roche, Avexis/Novartis Gene Therapies, Novartis, Biogen, and received consultancy fees from Roche, Novartis Gene Therapies, Biogen. LM received honoraria for speaking, advisory boards and compensation for congress participations from Sanofi Genzyme, Roche and Biogen. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Bautista-Herrera L. A., De la Cruz-Mosso U., Román-Fernández I. V., Parra-Rojas I., Soñanez-Organis J. G., Hernández-Bello J., et al. (2020). A potential inflammatory role of IL-31 in psoriatic arthritis: A correlation with Th17 cytokine profile. Int. J. Immunopathol. Pharmacol. 34:2058738420907186. 10.1177/2058738420907186 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

