Determination of ceftriaxone in human plasma using liquid chromatography-tandem mass spectrometry
- PMID: 36035377
- PMCID: PMC9379334
- DOI: 10.12688/wellcomeopenres.15141.3
Determination of ceftriaxone in human plasma using liquid chromatography-tandem mass spectrometry
Abstract
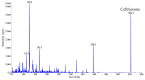
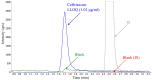
Ceftriaxone is a cephalosporin antibiotic drug used as first-line treatment for a number of bacterial diseases. Ceftriaxone belongs to the third generation of cephalosporin and is available as an intramuscular or intravenous injection. Previously published pharmacokinetic studies have used high-performance liquid chromatography coupled with ultraviolet detection (HPLC-UV) for the quantification of ceftriaxone. This study aimed to develop and validate a bioanalytical method for the quantification of ceftriaxone in human plasma using liquid chromatography followed by tandem mass spectrometry (LC-MS/MS). Sample preparation was performed by protein precipitation of 100 µl plasma sample in combination with phospholipid-removal techniques to minimize matrix interferences. The chromatographic separation was performed on an Agilent Zorbax Eclipse Plus C18 column with 10 mM ammonium formate containing 2% formic acid: acetonitrile as mobile phase at a flow rate of 0.4 ml/min with a total run time of 10 minutes. Both the analyte and cefotaxime (internal standard) were detected using the positive electrospray ionization (ESI) mode and selected reaction monitoring (SRM) for the precursor-product ion transitions m/z 555.0→396.1 for ceftriaxone and 456.0→324.0 for cefotaxime. The method was validated over the concentration range of 1.01-200 μg/ml. Calibration response showed good linearity (correlation coefficient > 0.99) and matrix effects were within the ±15% limit in 6 different lots of sodium heparin plasma tested. However, citrate phosphate dextrose plasma resulted in a clear matrix enhancement of 24% at the low concentration level, which was not compensated for by the internal standard. Different anticoagulants (EDTA, heparin and citrate phosphate dextrose) also showed differences in recovery. Thus, it is important to use the same anticoagulant in calibration curves and clinical samples for analysis. The intra-assay and inter-assay precision were less than 5% and 10%, respectively, and therefore well within standard regulatory acceptance criterion of ±15%.
Keywords: Ceftriaxone; bioanalytical method; human plasma; liquid chromatography tandem mass spectrometry.
Copyright: © 2022 Wongchang T et al.
Conflict of interest statement
No competing interests were disclosed.
Figures


References
-
- O’Neill J: Tackling drug-resistant infections globally: Final report and recommendations.2016;84. Date accessed: 4 Dec 2018. Reference Source
Associated data
LinkOut - more resources
Full Text Sources

