Rationale and design of the prevention of paclitaxel-related neurological side effects with lithium trial - Protocol of a multicenter, randomized, double-blind, placebo- controlled proof-of-concept phase-2 clinical trial
- PMID: 36035422
- PMCID: PMC9403739
- DOI: 10.3389/fmed.2022.967964
Rationale and design of the prevention of paclitaxel-related neurological side effects with lithium trial - Protocol of a multicenter, randomized, double-blind, placebo- controlled proof-of-concept phase-2 clinical trial
Abstract
Introduction: Chemotherapy-induced polyneuropathy (CIPN) and post-chemotherapy cognitive impairment (PCCI) are frequent side effects of paclitaxel treatment. CIPN/PCCI are potentially irreversible, reduce quality of life and often lead to treatment limitations, which affect patients' outcome. We previously demonstrated that paclitaxel enhances an interaction of the Neuronal calcium sensor-1 protein (NCS-1) with the Inositol-1,4,5-trisphosphate receptor (InsP3R), which disrupts calcium homeostasis and triggers neuronal cell death via the calcium-dependent protease calpain in dorsal root ganglia neurons and neuronal precursor cells. Prophylactic treatment of rodents with lithium inhibits the NCS1-InsP3R interaction and ameliorates paclitaxel-induced polyneuropathy and cognitive impairment, which is in part supported by limited retrospective clinical data in patients treated with lithium carbonate at the time of chemotherapy. Currently no data are available from a prospective clinical trial to demonstrate its efficacy.
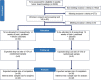
Methods and analysis: The PREPARE study will be conducted as a multicenter, randomized, double-blind, placebo-controlled phase-2 trial with parallel group design. N = 84 patients with breast cancer will be randomized 1:1 to either lithium carbonate treatment (targeted serum concentration 0.5-0.8 mmol/l) or placebo with sham dose adjustments as add-on to (nab-) paclitaxel. The primary endpoint is the validated Total Neuropathy Score reduced (TNSr) at 2 weeks after the last (nab-) paclitaxel infusion. The aim is to show that the lithium carbonate group is superior to the placebo group, meaning that the mean TNSr after (nab-) paclitaxel is lower in the lithium carbonate group than in the placebo group. Secondary endpoints include: (1) severity of CIPN, (2) amount and dose of pain medication, (3) cumulative dose of (nab-) paclitaxel, (4) patient-reported symptoms of CIPN, quality of life and symptoms of anxiety and depression, (5) severity of cognitive impairment, (6) hippocampal volume and changes in structural/functional connectivity and (7) serum Neurofilament light chain protein concentrations.
Ethics and dissemination: The study protocol was approved by the Berlin ethics committee (reference: 21/232 - IV E 10) and the respective federal agency (Bundesinstitut für Arzneimittel und Medizinprodukte, reference: 61-3910-4044771). The results of the study will be published in peer-reviewed medical journals as well as presented at relevant (inter)national conferences.
Clinical trial registration: [https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00027165], identifier [DRKS00027165].
Keywords: chemotherapy; cognitive impairment; lithium; neurotoxicity; paclitaxel; polyneuropathy; prevention.
Copyright © 2022 Huehnchen, Bangemann, Lischewski, Märschenz, Paul, Schmitz-Hübsch, Blohmer, Eberhardt, Rauch, Flöel, Adam, Schwenkenbecher, Meinhold-Heerlein, Hoffmann, Ziemssen, Endres and Boehmerle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol. (2007) 63:183–202. - PubMed
-
- Doth AH, Hansson PT, Jensen MP, Taylor RS. The burden of neuropathic pain: A systematic review and meta-analysis of health utilities. Pain. (2010) 149:338–44. - PubMed
LinkOut - more resources
Full Text Sources

