Light-Driven [FeFe] Hydrogenase Based H2 Production in E. coli: A Model Reaction for Exploring E. coli Based Semiartificial Photosynthetic Systems
- PMID: 36035441
- PMCID: PMC9400101
- DOI: 10.1021/acssuschemeng.2c03657
Light-Driven [FeFe] Hydrogenase Based H2 Production in E. coli: A Model Reaction for Exploring E. coli Based Semiartificial Photosynthetic Systems
Abstract
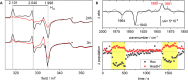
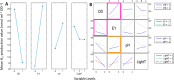
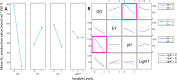
Biohybrid technologies like semiartificial photosynthesis are attracting increased attention, as they enable the combination of highly efficient synthetic light-harvesters with the self-healing and outstanding performance of biocatalysis. However, such systems are intrinsically complex, with multiple interacting components. Herein, we explore a whole-cell photocatalytic system for hydrogen (H2) gas production as a model system for semiartificial photosynthesis. The employed whole-cell photocatalytic system is based on Escherichia coli cells heterologously expressing a highly efficient, but oxygen-sensitive, [FeFe] hydrogenase. The system is driven by the organic photosensitizer eosin Y under broad-spectrum white light illumination. The direct involvement of the [FeFe] hydrogenase in the catalytic reaction is verified spectroscopically. We also observe that E. coli provides protection against O2 damage, underscoring the suitability of this host organism for oxygen-sensitive enzymes in the development of (photo) catalytic biohybrid systems. Moreover, the study shows how factorial experimental design combined with analysis of variance (ANOVA) can be employed to identify relevant variables, as well as their interconnectivity, on both overall catalytic performance and O2 tolerance.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Lorenzi M.; Berggren G.. 8.28 - [FeFe] Hydrogenases and Their Functional Models. In Comprehensive Coordination Chemistry III, Constable E. C., Parkin G., Que L. Jr., Eds.; Elsevier: Oxford, 2021; pp 731–756.
LinkOut - more resources
Full Text Sources
