Reproducing a decision-making network in a virtual visual discrimination task
- PMID: 36035443
- PMCID: PMC9399926
- DOI: 10.3389/fnint.2022.930326
Reproducing a decision-making network in a virtual visual discrimination task
Abstract
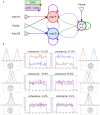
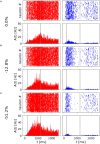
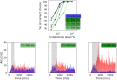
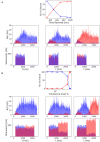
We reproduced a decision-making network model using the neural simulator software neural simulation tool (NEST), and we embedded the spiking neural network in a virtual robotic agent performing a simulated behavioral task. The present work builds upon the concept of replicability in neuroscience, preserving most of the computational properties in the initial model although employing a different software tool. The proposed implementation successfully obtains equivalent results from the original study, reproducing the salient features of the neural processes underlying a binary decision. Furthermore, the resulting network is able to control a robot performing an in silico visual discrimination task, the implementation of which is openly available on the EBRAINS infrastructure through the neuro robotics platform (NRP).
Keywords: NEST; decision-making; network model; neurorobot; reproducibility; working memory.
Copyright © 2022 Trapani, Sheiban, Bertone, Chiosso, Colombo, D'Andrea, De Santis, Fati, Fossati, Gonzalez and Pedrocchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Bradski G. (2000). The OpenCV Library. San Francisco, CA: Dr. Dobb's Journal of Software Tools.
LinkOut - more resources
Full Text Sources
Miscellaneous

