Leaf gas exchange and water relations of the woody desiccation-tolerant Paraboea rufescens during dehydration and rehydration
- PMID: 36035511
- PMCID: PMC9403483
- DOI: 10.1093/aobpla/plac033
Leaf gas exchange and water relations of the woody desiccation-tolerant Paraboea rufescens during dehydration and rehydration
Abstract
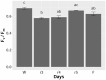
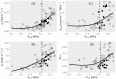
Desiccation-tolerant (DT) plants can withstand dehydration to less than 0.1 g H2O g-1 dry weight. The mechanism for whole-plant recovery from severe dehydration is still not clear, especially for woody DT plants. In the present study, we evaluated the desiccation tolerance and mechanism of recovery for a potentially new woody resurrection plant Paraboea rufescens (Gesneriaceae). We monitored the leaf water status, leaf gas exchange, chlorophyll fluorescence and root pressure of potted P. rufescens during dehydration and rehydration, and we investigated the water content and chlorophyll fluorescence of P. rufescens leaves in the field during the dry season. After re-watering from a severely dehydrated state, leaf maximum quantum yield of photosystem II of P. rufescens quickly recovered to well-watered levels. Leaf water status and leaf hydraulic conductance quickly recovered to well-watered levels after re-watering, while leaf gas exchange traits also trended to recovery, but at a slower rate. The maximum root pressure in rehydrated P. rufescens was more than twice in well-watered plants. Our study identified P. rufescens as a new DT woody plant. The whole-plant recovery of P. rufescens from extreme dehydration is potentially associated with an increase of root pressure after rehydration. These findings provide insights into the mechanisms of recovery of DT plants from dehydration.
Keywords: Desiccation tolerance; Gesneriaceae; Paraboea rufescens; leaf hydraulic conductance; rehydration; resurrection plant; root pressure.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures



References
-
- Alcantara S, de Mello-Silva R, Teodoro GS, Drequeceler K, Ackerly DD, Oliveira RS.. 2015. Carbon assimilation and habitat segregation in resurrection plants: a comparison between desiccation- and non-desiccation-tolerant species of Neotropical Velloziaceae (Pandanales). Functional Ecology 29:1499–1512.
-
- Alpert P. 2006. Constraints of tolerance: why are desiccation-tolerant organisms so small or rare? Journal of Experimental Biology 209:1575–1584. - PubMed
-
- Cao K-F, Yang S-J, Zhang Y-J, Brodribb TJ.. 2012. The maximum height of grasses is determined by roots. Ecology Letters. 15:666–672. - PubMed
-
- Deng X, Hu Z-A, Wang H-X, Wen X-G, Kuang T-Y.. 2003. A comparison of photosynthetic apparatus of the detached leaves of the resurrection plant Boea hygrometrica with its non-tolerant relative Chirita heterotrichia in response to dehydration and rehydration. Plant Science 165:851–861.
LinkOut - more resources
Full Text Sources

