The vertebrate Embryo Clock: Common players dancing to a different beat
- PMID: 36036002
- PMCID: PMC9403190
- DOI: 10.3389/fcell.2022.944016
The vertebrate Embryo Clock: Common players dancing to a different beat
Abstract
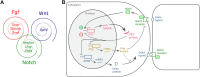
Vertebrate embryo somitogenesis is the earliest morphological manifestation of the characteristic patterned structure of the adult axial skeleton. Pairs of somites flanking the neural tube are formed periodically during early development, and the molecular mechanisms in temporal control of this early patterning event have been thoroughly studied. The discovery of a molecular Embryo Clock (EC) underlying the periodicity of somite formation shed light on the importance of gene expression dynamics for pattern formation. The EC is now known to be present in all vertebrate organisms studied and this mechanism was also described in limb development and stem cell differentiation. An outstanding question, however, remains unanswered: what sets the different EC paces observed in different organisms and tissues? This review aims to summarize the available knowledge regarding the pace of the EC, its regulation and experimental manipulation and to expose new questions that might help shed light on what is still to unveil.
Keywords: HES; embryo clock; negative feedback regulation; notch signalling; somitogenesis; temporal control.
Copyright © 2022 Carraco, Martins-Jesus and Andrade.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

