Natural history of liver disease in a large international cohort of children with Alagille syndrome: Results from the GALA study
- PMID: 36036223
- PMCID: PMC9869940
- DOI: 10.1002/hep.32761
Natural history of liver disease in a large international cohort of children with Alagille syndrome: Results from the GALA study
Abstract
Background and aims: Alagille syndrome (ALGS) is a multisystem disorder, characterized by cholestasis. Existing outcome data are largely derived from tertiary centers, and real-world data are lacking. This study aimed to elucidate the natural history of liver disease in a contemporary, international cohort of children with ALGS.
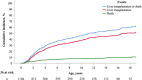
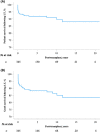
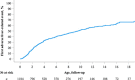
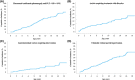
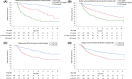
Approach and results: This was a multicenter retrospective study of children with a clinically and/or genetically confirmed ALGS diagnosis, born between January 1997 and August 2019. Native liver survival (NLS) and event-free survival rates were assessed. Cox models were constructed to identify early biochemical predictors of clinically evident portal hypertension (CEPH) and NLS. In total, 1433 children (57% male) from 67 centers in 29 countries were included. The 10 and 18-year NLS rates were 54.4% and 40.3%. By 10 and 18 years, 51.5% and 66.0% of children with ALGS experienced ≥1 adverse liver-related event (CEPH, transplant, or death). Children (>6 and ≤12 months) with median total bilirubin (TB) levels between ≥5.0 and <10.0 mg/dl had a 4.1-fold (95% confidence interval [CI], 1.6-10.8), and those ≥10.0 mg/dl had an 8.0-fold (95% CI, 3.4-18.4) increased risk of developing CEPH compared with those <5.0 mg/dl. Median TB levels between ≥5.0 and <10.0 mg/dl and >10.0 mg/dl were associated with a 4.8 (95% CI, 2.4-9.7) and 15.6 (95% CI, 8.7-28.2) increased risk of transplantation relative to <5.0 mg/dl. Median TB <5.0 mg/dl were associated with higher NLS rates relative to ≥5.0 mg/dl, with 79% reaching adulthood with native liver ( p < 0.001).
Conclusions: In this large international cohort of ALGS, only 40.3% of children reach adulthood with their native liver. A TB <5.0 mg/dl between 6 and 12 months of age is associated with better hepatic outcomes. These thresholds provide clinicians with an objective tool to assist with clinical decision-making and in the evaluation of therapies.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of American Association for the Study of Liver Diseases.
Conflict of interest statement
Binita M. Kamath consults for and received grants from Mirum and Albireo. She consults for Audentes Therapeutics and Third Rock Ventures. Bettina E. Hansen consults for Mirum, Albireo, Chemomab, Calliditas, Intercept and Cyma Bay. She received grants from Mirum, Albireo, Intercept and Cyma Bay. Deirdre A. Kelly consults for, advises, and received grants from Mirum and Albireo. Emmanuel M. Gonzales consults for Albireo, CTRS, Mirum, and Vivet. Henrik Arnell consults for Albireo and Baxter. Henry C. Lin advises Albireo. Emmanuel Jacquemin consults for Laboratoire CTRS France and Vivet Therapeutics France. Kathleen Schwarz consults for and received grants from Gilead. She consults for Mirum. She received grants from Albireo. Kathleen M. Loomes consults for and received grants from Albireo and Mirum. She consults for Travere Therapeutics. Lorenzo D'Antiga consults for Albireo and Mirum. M. Kyle Jensen consults for Albireo and Guidepoint Global. Nanda Kerkar advises Albireo and Mirum. Emanuele Nicastro advises and received grants from Mirum Pharmaceuticals. Noelle H. Ebel consults for Mirum Pharmaceuticals. Rene Romero consults for Albireo and Mirum. He received grants from Gilead. Richard J. Thompson owns stock in, consults for, and advises Generation Bio and Rectify. He consults for, advises, and is on the speakers' bureau for Albireo and Mirum. Rima Fawaz consults for Mirum. She advises Albireo. Ryan T. Fischer is on the speakers' bureau for Albireo and Mirum. Saul J. Karpen consults for Albireo, Intercept, and Mirum. Nancy B. Spinner consults for Mirum and Travere. Wikrom Karnsakul consults for and received grants from Albireo and Gilead. He consults for Mirum.
Figures

Comment in
-
The Global Alagille Alliance study: Redefining the natural history of Alagille syndrome.Hepatology. 2023 Feb 1;77(2):347-349. doi: 10.1002/hep.32760. Epub 2022 Sep 17. Hepatology. 2023. PMID: 36036191 No abstract available.
-
Letter to the Editor: Much more needed in natural history of Alagille syndrome.Hepatology. 2023 Apr 1;77(4):E78. doi: 10.1097/HEP.0000000000000006. Epub 2023 Feb 3. Hepatology. 2023. PMID: 36626631 No abstract available.
References
-
- Alagille D, Odièvre M, Gautier M, Dommergues JP. Hepatic ductular hypoplasia associated with characteristic facies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur. J Pediatr. 1975;86:63–71. - PubMed
-
- Alagille D, Estrada A, Hadchouel M, Gautier M, Odièvre M, Dommergues JP. Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr. 1987;110:195–200. - PubMed
-
- Emerick KM, Rand EB, Goldmuntz E, Krantz ID, Spinner NB, Piccoli DA. Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology. 1999;29:822–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

