Psychometric properties of kidney disease quality of life-36 (KDQOL-36) in dialysis patients in Indonesia
- PMID: 36036313
- PMCID: PMC9829614
- DOI: 10.1007/s11136-022-03236-6
Psychometric properties of kidney disease quality of life-36 (KDQOL-36) in dialysis patients in Indonesia
Abstract
Objective: The study aimed to evaluate the psychometric properties of KDQOL-36 Bahasa Indonesia in hemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD) patients in Indonesia.
Methods: The psychometric analysis was conducted in three hospitals offering both HD and CAPD. The validity was assessed through structural, convergent, and known-group validity, while reliability was evaluated using internal consistency and test-retest reliability.
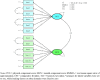
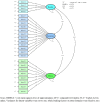
Results: The study involved 370 participants of which 71% received HD treatment. No floor and ceiling effects (< 10%) were identified. Confirmatory factor analysis supported a good model fit for both generic and kidney-specific domains, while exploratory factor analysis revealed three factors for kidney-specific domains and only three items with a loading factor below 0.4. Convergent validity showed positive correlations between kidney-specific domains, generic domains, and EQ-5D. The comparison of quality of life among subgroups based on dialysis type and whether or not patients had diabetes supported the hypotheses of known-group validity. Cronbach's alpha and omega values had demonstrated good internal consistency. Test-retest reliability indicated burden of kidney disease had good reliability, while other domains had moderate reliability.
Conclusion: The study supports the validity and reliability of both generic and kidney-specific domains of KDQOL-36 Bahasa Indonesia to evaluate quality of life in patients with HD and CAPD in Indonesia. As health-related quality of life is a crucial predictor of patient outcomes, this report contributes new evidence about validity and reliability to recommend the use of KDQOL-36 Bahasa Indonesia in dialysis centers.
Keywords: Indonesia; KDQOL; Quality of life; SF-12; Validation.
© 2022. The Author(s).
Conflict of interest statement
MJP reports grants and honoraria from various pharmaceutical companies, including those developing, producing and marketing diabetes drugs. However, all grants and honoraria were fully unrelated to this specific study. The other authors declare that they have no competing interests related to this specific study and topic.
Figures
References
-
- Wong CKH, Chen J, Fung SKS, Mok MMY, Cheng YL, Kong I, Lam CLK. Direct and indirect costs of end-stage renal disease patients in the first and second years after initiation of nocturnal home haemodialysis, hospital haemodialysis and peritoneal dialysis. Nephrology Dialysis Transplantation. 2019;34(9):1565–1576. doi: 10.1093/ndt/gfy395. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials