COVID-19 vaccination in peritoneal dialysis patients
- PMID: 36036315
- PMCID: PMC9421120
- DOI: 10.1007/s11255-022-03302-5
COVID-19 vaccination in peritoneal dialysis patients
Abstract
Background: COVID-19 vaccine is recommended in Peritoneal dialysis (PD) patients, but a paucity of data is available regarding vaccine-related adverse effects among PD patients.
Method: A cross-sectional study was conducted in a single center between October and November 2021. PD patients were provided with the online survey link to participate in the study.
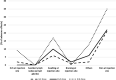
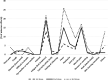
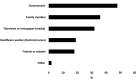
Results: A total of 107 PD patients responded to the survey (55%: male, 79%: Chinese, 40%: > 65 years old). Of these, 95% received the COVID-19 vaccine (77% received two doses and 22% received three doses). Most participants (91%) received Pfizer vaccine. The main source of vaccine information was from the government (48%). The most common reason to receive and refuse vaccines were the perception of the seriousness of COVID-19 infection (63%) and concern about vaccine safety (60%), respectively. After the first dose, 25% of patients developed one or more vaccine-related adverse effects. Common local adverse effect was pain at the injection site (21%), and systemic adverse effects were muscle pain (15%), fatigue (13%). Similar adverse effects were observed with subsequent doses. None of them required hospitalization for vaccine-related adverse effects. Female patients had a higher risk of developing adverse effects than male patients after the first dose (odds ratio: 3.37; 95% confidence interval: 1.25 - 9.08). No such difference was observed in the subsequent dose. Age, race, employment status and history of drug allergy were not associated with the risk of adverse effects.
Conclusions: The COVID-19 vaccine was well-tolerated by most PD patients, but few experienced non-severe adverse effects. All PD patients should be vaccinated against SAR-COV-2 infection.
Keywords: Adverse effects; COVID-19; End-stage kidney disease; Peritoneal dialysis; SAR-COV-2; Vaccine.
© 2022. The Author(s), under exclusive licence to Springer Nature B.V.
Conflict of interest statement
Htay Htay has received consultancy fees, speaker’s honoraria and travel sponsorships from Baxter Healthcare and consultancy fees and travel sponsorships from AWAK Technologies, speaker’s honoraria from Fresenius Medical Care, grants from Johnson & Johnson Company, grants from Singhealth NIG, outside the submitted work and Marjorie WY Foo has received grants from National Medical Research Council for the study; consultancy fees and speaker’s honoraria and travel sponsorships from Baxter Healthcare, consultancy fees and travel sponsorships from AWAK Technologies. The other authors have nothing to disclose.
Figures
Similar articles
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Antibody Response and Safety After mRNA-1273 SARS-CoV-2 Vaccination in Peritoneal Dialysis Patients - the Vienna Cohort.Front Immunol. 2021 Dec 2;12:780594. doi: 10.3389/fimmu.2021.780594. eCollection 2021. Front Immunol. 2021. PMID: 34925359 Free PMC article.
-
Safety and tolerability of the novel 2019 coronavirus disease (COVID-19) vaccines among people with epilepsy (PwE): A cross-sectional study.Seizure. 2021 Nov;92:2-9. doi: 10.1016/j.seizure.2021.08.001. Epub 2021 Aug 6. Seizure. 2021. PMID: 34391030 Free PMC article.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
-
Safety and effectiveness of vaccines against COVID-19 in children aged 5-11 years: a systematic review and meta-analysis.Lancet Child Adolesc Health. 2023 Jun;7(6):379-391. doi: 10.1016/S2352-4642(23)00078-0. Epub 2023 Apr 18. Lancet Child Adolesc Health. 2023. PMID: 37084750 Free PMC article.
Cited by
-
The Importance of Natural and Acquired Immunity to SARS-CoV-2 Infection in Patients on Peritoneal Dialysis.Vaccines (Basel). 2024 Jan 29;12(2):135. doi: 10.3390/vaccines12020135. Vaccines (Basel). 2024. PMID: 38400119 Free PMC article.
References
-
- Strategies regarding COVID-19 in PD patients - International Society for Peritoneal Dialysis [Internet]. [cited 2022 Feb 19]. Available from: https://ispd.org/strategies-covid19/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous