Regulation of Drought and Salt Tolerance by OsSKL2 and OsASR1 in Rice
- PMID: 36036369
- PMCID: PMC9424430
- DOI: 10.1186/s12284-022-00592-2
Regulation of Drought and Salt Tolerance by OsSKL2 and OsASR1 in Rice
Abstract
Abiotic stresses such as salinity and drought greatly impact the growth and production of crops worldwide. Here, a shikimate kinase-like 2 (SKL2) gene was cloned from rice and characterized for its regulatory function in salinity and drought tolerance. OsSKL2 was localized in the chloroplast, and its transcripts were significantly induced by drought and salinity stress as well as H2O2 and abscisic acid (ABA) treatment. Meanwhile, overexpression of OsSKL2 in rice increased tolerance to salinity, drought and oxidative stress by increasing antioxidant enzyme activity, and reducing levels of H2O2, malondialdehyde, and relative electrolyte leakage. In contrast, RNAi-induced suppression of OsSKL2 increased sensitivity to stress treatment. Interestingly, overexpression of OsSKL2 also increased sensitivity to exogenous ABA, with an increase in reactive oxygen species (ROS) accumulation. Moreover, OsSKL2 was found to physically interact with OsASR1, a well-known chaperone-like protein, which also exhibited positive roles in salt and drought tolerance. A reduction in ROS production was also observed in leaves of Nicotiana benthamiana showing transient co-expression of OsSKL2 with OsASR1. Taken together, these findings suggest that OsSKL2 together with OsASR1 act as important regulatory factors that confer salt and drought tolerance in rice via ROS scavenging.
Keywords: Oryza sativa; OsASR1; OsSKL2; Protein interactions; Reactive oxygen species; Salt and drought tolerance.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


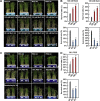
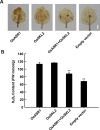
Similar articles
-
ASR Enhances Environmental Stress Tolerance and Improves Grain Yield by Modulating Stomatal Closure in Rice.Front Plant Sci. 2020 Feb 14;10:1752. doi: 10.3389/fpls.2019.01752. eCollection 2019. Front Plant Sci. 2020. PMID: 32117337 Free PMC article.
-
The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production.Plant Cell Physiol. 2014 Dec;55(12):2060-76. doi: 10.1093/pcp/pcu133. Epub 2014 Sep 26. Plant Cell Physiol. 2014. PMID: 25261532
-
Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA-mediated regulatory pathway and ROS scavenging.PLoS Genet. 2018 Oct 10;14(10):e1007662. doi: 10.1371/journal.pgen.1007662. eCollection 2018 Oct. PLoS Genet. 2018. PMID: 30303953 Free PMC article.
-
OsIAA18, an Aux/IAA Transcription Factor Gene, Is Involved in Salt and Drought Tolerance in Rice.Front Plant Sci. 2021 Nov 18;12:738660. doi: 10.3389/fpls.2021.738660. eCollection 2021. Front Plant Sci. 2021. PMID: 34868122 Free PMC article.
-
The Role of Anthocyanins in Plant Tolerance to Drought and Salt Stresses.Plants (Basel). 2023 Jul 5;12(13):2558. doi: 10.3390/plants12132558. Plants (Basel). 2023. PMID: 37447119 Free PMC article. Review.
Cited by
-
The Emerging Role of Non-Coding RNAs (ncRNAs) in Plant Growth, Development, and Stress Response Signaling.Noncoding RNA. 2024 Feb 7;10(1):13. doi: 10.3390/ncrna10010013. Noncoding RNA. 2024. PMID: 38392968 Free PMC article. Review.
-
Comprehensive characterization of poplar HSP20 gene family: genome-wide identification, stress-induced expression profiling, and protein interaction verifications.BMC Plant Biol. 2025 Feb 25;25(1):251. doi: 10.1186/s12870-025-06264-9. BMC Plant Biol. 2025. PMID: 39994524 Free PMC article.
-
Physiological and molecular implications of multiple abiotic stresses on yield and quality of rice.Front Plant Sci. 2023 Jan 11;13:996514. doi: 10.3389/fpls.2022.996514. eCollection 2022. Front Plant Sci. 2023. PMID: 36714754 Free PMC article. Review.
-
A Virulence Factor from Sclerotinia sclerotiorum Targets the Host Chloroplast Proteins to Promote Infection.Plants (Basel). 2024 Dec 6;13(23):3430. doi: 10.3390/plants13233430. Plants (Basel). 2024. PMID: 39683223 Free PMC article.
-
The analysis of the genetic loci affecting phenotypic plasticity of soybean isoflavone content by dQTG.seq model.Theor Appl Genet. 2024 Dec 17;138(1):9. doi: 10.1007/s00122-024-04798-4. Theor Appl Genet. 2024. PMID: 39688708
References
-
- Achary VMM, Sheri V, Manna M, Panditi B, Ram B, Agarwal A, Fartyal D, Teotia D, Masakapalli SK, Agrawal PK, Reddy MK. Overexpression of improved EPSPS gene results in field level glyphosate tolerance and higher grain yield in rice. Plant Biotechnol J. 2020;18(12):2504–2519. doi: 10.1111/pbi.13428. - DOI - PMC - PubMed
-
- Arenhart RA, Bai Y, Oliveira LFV, Neto LB, Schunemann M, Maraschin Fdos S, Mariath J, Silverio A, Sachetto-Martins G, Margis R, Wang ZY, Margis-Pinheiro M. New insights into aluminum tolerance in rice: the ASR5 protein binds the STAR1 promoter and other aluminum responsive genes. Mol Plant. 2014;7:709–721. doi: 10.1093/mp/sst160. - DOI - PMC - PubMed
-
- Azevedo-Neto AD, Prisco JT, Enéas-Filho J, Abreu CEB, Gomes-Filho E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot. 2006;56:87–94. doi: 10.1016/j.envexpbot.2005.01.008. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

