Skeletal muscle blood flow during exercise is reduced in a rat model of pulmonary hypertension
- PMID: 36036455
- PMCID: PMC9602702
- DOI: 10.1152/ajpregu.00327.2021
Skeletal muscle blood flow during exercise is reduced in a rat model of pulmonary hypertension
Abstract
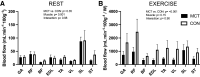
Pulmonary arterial hypertension (PAH) is characterized by exercise intolerance. Muscle blood flow may be reduced during exercise in PAH; however, this has not been directly measured. Therefore, we investigated blood flow during exercise in a rat model of monocrotaline (MCT)-induced pulmonary hypertension (PH). Male Sprague-Dawley rats (∼200 g) were injected with 60 mg/kg MCT (MCT, n = 23) and vehicle control (saline; CON, n = 16). Maximal rate of oxygen consumption (V̇o2max) and voluntary running were measured before PH induction. Right ventricle (RV) morphology and function were assessed via echocardiography and invasive hemodynamic measures. Treadmill running at 50% V̇o2max was performed by a subgroup of rats (MCT, n = 8; CON, n = 7). Injection of fluorescent microspheres determined muscle blood flow via photo spectroscopy. MCT demonstrated a severe phenotype via RV hypertrophy (Fulton index, 0.61 vs. 0.31; P < 0.001), high RV systolic pressure (51.5 vs. 22.4 mmHg; P < 0.001), and lower V̇o2max (53.2 vs. 71.8 mL·min-1·kg-1; P < 0.0001) compared with CON. Two-way ANOVA revealed exercising skeletal muscle blood flow relative to power output was reduced in MCT compared with CON (P < 0.001), and plasma lactate was increased in MCT (10.8 vs. 4.5 mmol/L; P = 0.002). Significant relationships between skeletal blood flow and blood lactate during exercise were observed for individual muscles (r = -0.58 to -0.74; P < 0.05). No differences in capillarization were identified. Skeletal muscle blood flow is significantly reduced in experimental PH. Reduced blood flow during exercise may be, at least in part, consequent to reduced exercise intensity in PH. This adds further evidence of peripheral muscle dysfunction and exercise intolerance in PAH.
Keywords: PAH; blood flow; exercise; pulmonary hypertension; skeletal muscle.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





Comment in
-
Skeletal muscle blood flow during exercise is reduced in a rat model of pulmonary hypertension.Am J Physiol Regul Integr Comp Physiol. 2023 Jun 1;324(6):R772-R773. doi: 10.1152/ajpregu.00009.2023. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 37255499 No abstract available.
References
-
- Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, Picard F, de Groote P, Jevnikar M, Bergot E, Chaouat A, Chabanne C, Bourdin A, Parent F, Montani D, Simonneau G, Humbert M, Sitbon O. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 50: 1700889, 2017. doi:10.1183/13993003.00889-2017. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

