Dysfunctional Glymphatic System with Disrupted Aquaporin 4 Expression Pattern on Astrocytes Causes Bacterial Product Accumulation in the CSF during Pneumococcal Meningitis
- PMID: 36036510
- PMCID: PMC9600563
- DOI: 10.1128/mbio.01886-22
Dysfunctional Glymphatic System with Disrupted Aquaporin 4 Expression Pattern on Astrocytes Causes Bacterial Product Accumulation in the CSF during Pneumococcal Meningitis
Abstract
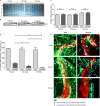
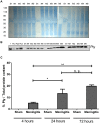
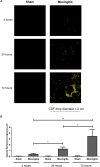
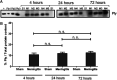
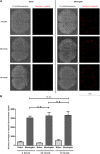
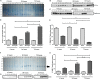
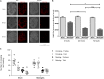
Pneumococcal meningitis, inflammation of the meninges due to an infection of the Central Nervous System caused by Streptococcus pneumoniae (the pneumococcus), is the most common form of community-acquired bacterial meningitis globally. Aquaporin 4 (AQP4) water channels on astrocytic end feet regulate the solute transport of the glymphatic system, facilitating the exchange of compounds between the brain parenchyma and the cerebrospinal fluid (CSF), which is important for the clearance of waste away from the brain. Wistar rats, subjected to either pneumococcal meningitis or artificial CSF (sham control), received Evans blue-albumin (EBA) intracisternally. Overall, the meningitis group presented a significant impairment of the glymphatic system by retaining the EBA in the CSF compartments compared to the uninfected sham group. Our results clearly showed that during pneumococcal meningitis, the glymphatic system does not function because of a detachment of the astrocytic end feet from the blood-brain barrier (BBB) vascular endothelium, which leads to misplacement of AQP4 with the consequent loss of the AQP4 water channel's functionality. IMPORTANCE The lack of solute drainage due to a dysfunctional glymphatic system leads to an increase of the neurotoxic bacterial material in the CSF compartments of the brain, ultimately leading to brain-wide neuroinflammation and neuronal damage with consequent impairment of neurological functions. The loss of function of the glymphatic system can therefore be a leading cause of the neurological sequelae developing post-bacterial meningitis.
Keywords: Streptococcus pneumoniae; aquaporin 4; astrocytic end feet; blood-brain barrier (BBB); glymphatic system; meningitis; neuroinflammation; neuronal damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
The Glymphatic System: a Potential Key Player in Bacterial Meningitis.mBio. 2022 Dec 20;13(6):e0235022. doi: 10.1128/mbio.02350-22. Epub 2022 Oct 26. mBio. 2022. PMID: 36286550 Free PMC article.
Similar articles
-
Aquaporin-4-dependent glymphatic solute transport in the rodent brain.Elife. 2018 Dec 18;7:e40070. doi: 10.7554/eLife.40070. Elife. 2018. PMID: 30561329 Free PMC article.
-
Loss of aquaporin-4 results in glymphatic system dysfunction via brain-wide interstitial fluid stagnation.Elife. 2023 Feb 9;12:e82232. doi: 10.7554/eLife.82232. Elife. 2023. PMID: 36757363 Free PMC article.
-
Circadian control of brain glymphatic and lymphatic fluid flow.Nat Commun. 2020 Sep 2;11(1):4411. doi: 10.1038/s41467-020-18115-2. Nat Commun. 2020. PMID: 32879313 Free PMC article.
-
Astrocytes, HIV and the Glymphatic System: A Disease of Disrupted Waste Management?Front Cell Infect Microbiol. 2020 Sep 29;10:523379. doi: 10.3389/fcimb.2020.523379. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33134185 Free PMC article. Review.
-
The role of glymphatic system in the cerebral edema formation after ischemic stroke.Exp Neurol. 2021 Jun;340:113685. doi: 10.1016/j.expneurol.2021.113685. Epub 2021 Mar 5. Exp Neurol. 2021. PMID: 33676917 Review.
Cited by
-
Neurochemical effects of sepsis on the brain.Clin Sci (Lond). 2023 Mar 31;137(6):401-414. doi: 10.1042/CS20220549. Clin Sci (Lond). 2023. PMID: 36942500 Free PMC article.
-
Intraventricular dimethyl sulfoxide (DMSO) induces hydrocephalus in a dose-dependent pattern.Heliyon. 2024 Mar 4;10(5):e27295. doi: 10.1016/j.heliyon.2024.e27295. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38486744 Free PMC article.
-
The Flipons, Infections, and Amyloids that Foreshadow the Fading Memories of Alzheimer's Disease.Neurosci Insights. 2025 Jun 6;20:26331055251338815. doi: 10.1177/26331055251338815. eCollection 2025. Neurosci Insights. 2025. PMID: 40486248 Free PMC article. Review.
-
Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood-Brain Barrier.Cells. 2024 Jan 12;13(2):150. doi: 10.3390/cells13020150. Cells. 2024. PMID: 38247841 Free PMC article. Review.
-
Glymphatic system: a gateway for neuroinflammation.Neural Regen Res. 2024 Dec 1;19(12):2661-2672. doi: 10.4103/1673-5374.391312. Epub 2023 Dec 21. Neural Regen Res. 2024. PMID: 38595285 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

