Comparison of the SARS-CoV-2 BD Veritor Nasal Antigen Test with Nasopharyngeal Reverse Transcription-PCR in Symptomatic Patients
- PMID: 36036635
- PMCID: PMC9603274
- DOI: 10.1128/spectrum.00190-22
Comparison of the SARS-CoV-2 BD Veritor Nasal Antigen Test with Nasopharyngeal Reverse Transcription-PCR in Symptomatic Patients
Abstract
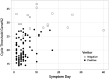
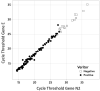
This study evaluated the BD Veritor system for rapid detection of SARS-CoV-2, an immunochromatographic point-of-care test, by comparing it with a standard reverse transcription-PCR (RT-PCR) methodology using samples from symptomatic patients. Samples from 146 symptomatic and 2 asymptomatic patients between the 1st and the 40th day of infection were evaluated. The nasopharyngeal and/or oropharyngeal swabs were inserted in a tube containing 0.9% saline solution and stored at refrigerator temperature until the moment of use. The samples were first tested with the Xpert Xpress SARS-CoV-2 (GeneXpert) kit (RT-PCR method), and the cycle thresholds (CTs) for the E and N2 genes encoding the SARS-CoV-2 envelope and nucleoprotein, respectively, were established. Subsequently, the same samples were tested using the Veritor rapid test. We analyzed the CTs of the N2 gene, which is detected in both methodologies, and observed sensitivities of 100%, 98.8%, 89.6%, and 82.7% for the CTs of <25, <27, and <30 and all the CTs, respectively. The greatest sensitivity was observed when we performed the test on patients within 5 days of symptom onset. The BD Veritor system's workflow is simple and fast, taking approximately 16 min from sample preparation to obtaining the test result. In addition to its satisfactory sensitivity, with results that correlate with those of the RT-PCR, the BD Veritor analyzer instrument reduces the subjectivity of unaided visual readings and consequent potential variation in result interpretation. Therefore, our results showed that the BD Veritor diagnostic test can provide a rapid and accurate diagnosis for SARS-CoV-2. IMPORTANCE This study provides important and useful information, especially for diagnostic laboratories, since the results show that the BD Veritor system can provide a fast and safe point-of-care antigen diagnostic test for rapid detection of COVID-19 that has high sensitivity, reproducibility, and accuracy.
Keywords: COVID-19; POC test; RT-PCR; SARS-CoV-2; Veritor test; coronavirus; immunochromatographic; point-of-care test; point-of-care testing; rapid antigen test; rapid tests.
Conflict of interest statement
The authors declare a conflict of interest. J.C., T.B., L.G., and G.L. are employees of Becton, Dickinson and Company; P.K., M. Maluf, F.N., M. Martino none.
Figures

References
-
- World Health Organization. 2020. Severe acute respiratory syndrome (SARS). World Health Organization, Geneva, Switzerland. https://www.who.int/csr/sars/en/.
-
- World Health Organization. 2020. IHR procedures concerning public health emergencies of international concern (PHEIC). World Health Organization, Geneva, Switzerland. Accessed 20 May 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

