Atomic Structure of IglD Demonstrates Its Role as a Component of the Baseplate Complex of the Francisella Type VI Secretion System
- PMID: 36036641
- PMCID: PMC9600919
- DOI: 10.1128/mbio.01277-22
Atomic Structure of IglD Demonstrates Its Role as a Component of the Baseplate Complex of the Francisella Type VI Secretion System
Abstract
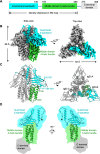
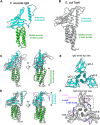
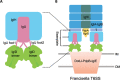
Francisella tularensis, a Tier 1 select agent of bioterrorism, contains a type VI secretion system (T6SS) encoded within the Francisella pathogenicity island (FPI), which is critical for its pathogenesis. Among the 18 proteins encoded by FPI is IglD, which is essential to Francisella's intracellular growth and virulence, but neither its location within T6SS nor its functional role has been established. Here, we present the cryoEM structure of IglD from Francisella novicida and show that the Francisella IglD forms a homotrimer that is structurally homologous to the T6SS baseplate protein TssK in Escherichia coli. Each IglD monomer consists of an N-terminal β-sandwich domain, a 4-helix bundle domain, and a flexible C-terminal domain. While the overall folds of IglD and TssK are similar, the two structures differ in three aspects: the relative orientation between their β-sandwich and the 4-helix bundle domains; two insertion loops present in TssK's β-sandwich domain; and, consequently, a lack of subunit-subunit interaction between insertion loops in the IglD trimer. Phylogenetic analysis indicates that IglD is genetically remote from the TssK orthologs in other T6SSs. While the other components of the Francisella baseplate are unknown, we conducted pulldown assays showing IglJ interacts with IglD and IglH, pointing to a model wherein IglD, IglH, and IglJ form the baseplate of the Francisella T6SS. Alanine substitution mutagenesis further established that IglD's hydrophobic pocket in the N-terminal β-sandwich domain interacts with two loops of IglJ, reminiscent of the TssK-TssG interaction. These results form a framework for understanding the hitherto unexplored Francisella T6SS baseplate. IMPORTANCE Francisella tularensis is a facultatively intracellular Gram-negative bacterium that causes the serious and potentially fatal zoonotic illness, tularemia. Because of its extraordinarily high infectivity and mortality to humans, especially when inhaled, F. tularensis is considered a potential bioterrorism agent and is classified as a Tier 1 select agent. The type VI secretion system (T6SS) encoded within the Francisella pathogenicity island (FPI) is critical to its pathogenesis, but its baseplate components are largely unknown. Here, we report the cryoEM structure of IglD from Francisella novicida and demonstrate its role as a component of the baseplate complex of the Francisella T6SS. We further show that IglD interacts with IglJ and IglH, and propose a model in which these proteins interact to form the Francisella T6SS baseplate. Elucidation of the structure and composition of the Francisella baseplate should facilitate the design of strategies to prevent and treat infections caused by F. tularensis.
Keywords: Francisella; IglD; baseplate complex; contractile injection system; cryo-electron microscopy; intracellular pathogen; type VI secretion system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

