Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST-AFNET 4 trial
- PMID: 36036648
- PMCID: PMC9584752
- DOI: 10.1093/eurheartj/ehac471
Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST-AFNET 4 trial
Abstract
Aims: A strategy of systematic, early rhythm control (ERC) improves cardiovascular outcomes in patients with atrial fibrillation (AF). It is not known how this outcome-reducing effect is mediated.
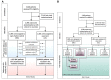
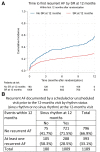
Methods and results: Using the Early treatment of Atrial Fibrillation for Stroke prevention Trial (EAST-AFNET 4) data set, potential mediators of the effect of ERC were identified in the total study population at 12-month follow up and further interrogated by use of a four-way decomposition of the treatment effect in an exponential model predicting future primary outcome events. Fourteen potential mediators of ERC were identified at the 12-month visit. Of these, sinus rhythm at 12 months explained 81% of the treatment effect of ERC compared with usual care during the remainder of follow up (4.1 years). In patients not in sinus rhythm at 12 months, ERC did not reduce future cardiovascular outcomes (hazard ratio 0.94, 95% confidence interval 0.65-1.67). Inclusion of AF recurrence in the model only explained 31% of the treatment effect, and inclusion of systolic blood pressure at 12 months only 10%. There was no difference in outcomes in patients who underwent AF ablation compared with those who did not undergo AF ablation.
Conclusion: The effectiveness of early rhythm control is mediated by the presence of sinus rhythm at 12 months in the EAST-AFNET 4 trial. Clinicians implementing ERC should aim for rapid and sustained restoration of sinus rhythm in patients with recently diagnosed AF and cardiovascular comorbidities.
Trial registration: ClinicalTrials.gov NCT01288352.
Keywords: AF ablation; Antiarrhythmic drugs; Atrial fibrillation; Mediator analysis; Randomized trials; Rhythm control; Stroke.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology.
Conflict of interest statement
Conflict of interest: L.E. discloses consultant fees, speaking honoraria, and travel expenses from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boehringer, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, and Sanofi Aventis. Research has been supported by German Research Foundation (DFG) and German Heart Foundation outside the submitted work. S.S. reports grants from AFNET, during the conduct of the study; grants from Biotronik, personal fees from Boston Scientific and grants from ResMed, outside the submitted work. A.S. reports grants from AFNET, during the conduct of the study; grants from BIOTRONIK and grants from ResMed, outside the submitted work. K.B. declares that there is no conflict of interest. G.B. reports no potential conflicts of interest with regard to the present substudy of EAST. H.J.G.M.C. reports support from The Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation, CVON 2014-9: reappraisal of atrial fibrillation: interaction between hypercoagulability, electrical remodelling, and vascular destabilization in the progression of AF (RACE V), outside this work. A.G. reports speaker and consulting fees from Bayer Health Care, Berlin Chemie, Boehringer Ingelheim, BMS/Pfizer, Boston Scientific, Daiichi Sankyo, Medtronic, Omeicos, and Menarini; grants from EU Horizon 2020 [grant no. 965286] and grants from AFNET, Sanofia-Aventis, and St Jude Medical during the conduct of the study. K.W. reports grants from AFNET, during the conduct of the study; grants from Biotronik, personal fees from Biotronik, personal fees from Boston Scientific, grants from ResMed and personal fees from Novartis, outside the submitted work. A.Z. reports grants from AFNET, during the conduct of the study; grants from Biotronik, personal fees from Boston Scientific and grants from ResMed, outside the submitted work. A.J.C. reports personal fees from Abbott and Sanofi and institutional grants from Abbott for work unrelated to the EAST trial. A.M. reports consulting fees from Medtronic and Biosense Webster; speaker fees from Medtronic, Biosense Webster, Boston Scientific, and Bayer; travel support from Medtronic and Boston Scientific. P.K. reports grants and nonfinancial support from BMBF (German Ministry of Education and Research), grants from Sanofi, grants from Abbott, grants and nonfinancial support from EHRA, grants from German Heart Foundation, grants from DZHK (German Center for Cardiovascular Research), during the conduct of the study; grants from European Union, grants from British Heart Foundation, grants from Leducq Foundation, grants from Medical Research Council (UK), non-financial support from German Centre for Heart Research, outside the submitted work. In addition, P.K. has a patent Atrial Fibrillation Therapy WO 2015140571 issued to University of Birmingham, and a patent Markers for Atrial Fibrillation WO 2016012783 issued to University of Birmingham.
Figures




Comment in
-
Sinus rhythm: the sine qua non for rhythm control?Eur Heart J. 2022 Oct 21;43(40):4145-4147. doi: 10.1093/eurheartj/ehac490. Eur Heart J. 2022. PMID: 36036652 No abstract available.
References
-
- Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–1316. - PubMed
-
- Goette A, Borof K, Breithardt G, Camm A, Crijns H, Kuck K, et al. Presenting pattern of atrial fibrillation and outcomes of early rhythm control therapy. J Am Coll Cardiol 2022;80:283–295. - PubMed

