Empagliflozin in acute myocardial infarction: the EMMY trial
- PMID: 36036746
- PMCID: PMC9622301
- DOI: 10.1093/eurheartj/ehac494
Empagliflozin in acute myocardial infarction: the EMMY trial
Abstract
Aims: Sodium-glucose co-transporter 2 inhibition reduces the risk of hospitalization for heart failure and for death in patients with symptomatic heart failure. However, trials investigating the effects of this drug class in patients following acute myocardial infarction are lacking.
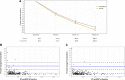
Methods and results: In this academic, multicentre, double-blind trial, patients (n = 476) with acute myocardial infarction accompanied by a large creatine kinase elevation (>800 IU/L) were randomly assigned to empagliflozin 10 mg or matching placebo once daily within 72 h of percutaneous coronary intervention. The primary outcome was the N-terminal pro-hormone of brain natriuretic peptide (NT-proBNP) change over 26 weeks. Secondary outcomes included changes in echocardiographic parameters. Baseline median (interquartile range) NT-proBNP was 1294 (757-2246) pg/mL. NT-proBNP reduction was significantly greater in the empagliflozin group, compared with placebo, being 15% lower [95% confidence interval (CI) -4.4% to -23.6%] after adjusting for baseline NT-proBNP, sex, and diabetes status (P = 0.026). Absolute left-ventricular ejection fraction improvement was significantly greater (1.5%, 95% CI 0.2-2.9%, P = 0.029), mean E/e' reduction was 6.8% (95% CI 1.3-11.3%, P = 0.015) greater, and left-ventricular end-systolic and end-diastolic volumes were lower by 7.5 mL (95% CI 3.4-11.5 mL, P = 0.0003) and 9.7 mL (95% CI 3.7-15.7 mL, P = 0.0015), respectively, in the empagliflozin group, compared with placebo. Seven patients were hospitalized for heart failure (three in the empagliflozin group). Other predefined serious adverse events were rare and did not differ significantly between groups.
Conclusion: In patients with a recent myocardial infarction, empagliflozin was associated with a significantly greater NT-proBNP reduction over 26 weeks, accompanied by a significant improvement in echocardiographic functional and structural parameters.
Clinicaltrials.gov registration: NCT03087773.
Keywords: Clinical trial; Empagliflozin; Heart failure; Myocardial infarction; NT-proBNP; Randomised controlled trial.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology.
Conflict of interest statement
Conflict of interest: H.S. is on the advisory board and speakers bureau of by Boehringer Ingelheim, NovoNordisk, Sanofi-Aventis, Amgen, AstraZeneca, Bayer, Eli Lilly, Kapsch, MSD, and Daiichi Sankyo. D.V.L. is on the advisory board and speakers’ bureau of Abiomed, AstraZeneca, Bayer, Daiichi Sankyo, Orion, Sanofi, and Servier and receives consulting fees from Recardio Inc, Bayer, TLL, Vaxxinity Inc. P.M.Z. received speaker fees from AstraZeneca, Pfizer, Medtronic, and Boehringer Ingelheim. R.R.H. reports research support from AstraZeneca, Bayer and Merck Sharp & Dohme, and personal fees from Anji Pharmaceuticals, AstraZeneca, Novartis, and Novo Nordisk. M.W. receives speaker fees from Bayer, Novartis and consulting fees from Radcliff Cardiology. A.Z. receives fees from Boehringer Ingelheim, AstraZeneca, NovoNordisk, Bayer, Daiichi Sankyo, Amgen, and Sanofi. C.R. receives payment or Honoria for lectures from Boehringer Ingelheim, Pfizer, and AstraZeneca and support for attending meetings from Boehringer Ingelheim and Pfizer. T.R.P. receives consulting fees and payment of honoraria for lectures from Arecor and Novo Nordisk and grants from Arecor, Sanofi, and Novo Nordisk. H.A. receives Honoria for lectures from Boehringer Ingelheim and AstraZeneca and financial support for attending meetings and transport from Boehringer Ingelheim and AstraZeneca. P.A. reports speakers’ fee from Bayer and Pfizer. J.M.S.M. received speaker or consultant fees from Chiesi, Boehringer Ingelheim, Biosensors, P&F, Gruenenthal, Bayer, Medtronic, and Boston Scientific within the last 3 years. D.M. receives consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, and Vifor, further he receives payment for lectures from AstraZeneca, Bayer, Boehringer Ingelheim, Vifor, and BMS. C.H.S. reports grants and consulting fees from AstraZeneca, Boehringer Ingelheim, and MSD. M.L. receives consulting fees from Johnson and Johnson and support for attending meetings from MSD. R.B. received a grant from Abbott and speaker or consultant fees from Abbott, Amgen, AstraZeneca, Biotronik, Bayer, Boehringer Ingelheim, Novartis, and Vivorpharma within the last 3 years. The remaining authors have no relevant conflict of interest.
Figures


Comment in
-
Putting the puzzle together: SGLT2 inhibitors from prevention to treatment of heart failure.Eur Heart J. 2022 Nov 1;43(41):4433-4435. doi: 10.1093/eurheartj/ehac483. Eur Heart J. 2022. PMID: 36036745 No abstract available.
-
SGLT2 inhibition could potentially impact inflammation in acute myocardial infarction.Eur Heart J. 2023 Oct 12;44(38):3931. doi: 10.1093/eurheartj/ehad404. Eur Heart J. 2023. PMID: 37350395 No abstract available.
References
-
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. - PubMed
-
- Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 Inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet 2020;396:819–829. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

