Regioselective α-Cyanation of Unprotected Alicyclic Amines
- PMID: 36036764
- PMCID: PMC9548390
- DOI: 10.1021/acs.orglett.2c02148
Regioselective α-Cyanation of Unprotected Alicyclic Amines
Abstract
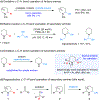
Secondary alicyclic amines are converted to α-aminonitriles via addition of TMSCN to their corresponding imines, intermediates that are produced in situ via the oxidation of amine-derived lithium amides with simple ketone oxidants. Amines with an existing α-substituent undergo regioselective α'-cyanation even if the C-H bonds at that site are less activated. Amine α-arylation can be combined with α'-cyanation to generate difunctionalized products in a single operation.
Figures

References
-
- Relevance of azacycles in medicine:Taylor RD; MacCoss M; Lawson ADG, Rings in Drugs. J. Med. Chem 2014, 57, 5845–5859; - PubMed
- Vitaku E; Smith DT; Njardarson JT, Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. - PubMed
-
- For a general overview of amine C─H bond functionalization, see:Dutta S; Li B; Rickertsen DRL; Valles DA; Seidel D, C─H Bond Functionalization of Amines: A Graphical Overview of Diverse Methods. SynOpen 2021, 05, 173–228. - PMC - PubMed
- Other selected reviews: Campos KR, Direct sp3 C-H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev 2007, 36, 1069–1084; - PubMed
- Jazzar R; Hitce J; Renaudat A; Sofack-Kreutzer J; Baudoin O, Functionalization of Organic Molecules by Transition-Metal-Catalyzed C(sp3)-H Activation. Chem. Eur. J 2010, 16, 2654–2672; - PubMed
- Mitchell EA; Peschiulli A; Lefevre N; Meerpoel L; Maes BUW, Direct alpha-Functionalization of Saturated Cyclic Amines. Chem. Eur. J 2012, 18, 10092–10142; - PubMed
- Peng B; Maulide N, The Redox-Neutral Approach to C-H Functionalization. Chem. Eur. J 2013, 19, 13274–13287; - PubMed
- Girard SA; Knauber T; Li C-J, The Cross-Dehydrogenative Coupling of C sp3-H Bonds: A Versatile Strategy for C-C Bond Formations. Angew. Chem. Int. Ed 2014, 53, 74–100; - PubMed
- Haibach MC; Seidel D, C-H Bond Functionalization through Intramolecular Hydride Transfer. Angew. Chem. Int. Ed 2014, 53, 5010–5036; - PubMed
- Beatty JW; Stephenson CRJ, Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis. Acc. Chem. Res 2015, 48, 1474–1484; - PMC - PubMed
- Cheng M-X; Yang S-D, Recent Advances in the Enantioselective Oxidative α-C─H Functionalization of Amines. Synlett 2017, 28, 159–174;
- Chu JCK; Rovis T, Complementary Strategies for Directed C(sp3)─H Functionalization: A Comparison of Transition-Metal-Catalyzed Activation, Hydrogen Atom Transfer, and Carbene/Nitrene Transfer. Angew. Chem. Int. Ed 2018, 57, 62–101; - PMC - PubMed
- Stateman LM; Nakafuku KM; Nagib DA, Remote C─H Functionalization via Selective Hydrogen Atom Transfer. Synthesis 2018, 50, 1569–1586; - PMC - PubMed
- Edwards PM; Schafer LL, Early transition metal-catalyzed C─H alkylation: hydroaminoalkylation for Csp3─Csp3 bond formation in the synthesis of selectively substituted amines. Chem. Commun 2018, 54, 12543–12560; - PubMed
- Gonnard L; Guérinot A; Cossy J, Transition metal-catalyzed α-alkylation of amines by C(sp3)─H bond activation. Tetrahedron 2019, 75, 145–163;
- Liu S; Zhao Z; Wang Y, Construction of N-Heterocycles through Cyclization of Tertiary Amines. Chem. Eur. J 2019, 25, 2423–2441; - PubMed
- Antermite D; Bull JA, Transition Metal-Catalyzed Directed C(sp3)─H Functionalization of Saturated Heterocycles. Synthesis 2019, 51, 3171–3204;
- Zhang T; Wu Y-H; Wang N-X; Xing Y, Advances in C(sp3)─H Bond Functionalization via Radical Processes. Synthesis 2019, 51, 4531–4548;
- Trowbridge A; Walton SM; Gaunt MJ, New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev 2020, 120, 2613–2692; - PubMed
- Kapoor M; Singh A; Sharma K; Hua Hsu M, Site-Selective C(sp3)─H and C(sp2)─H Functionalization of Amines Using a Directing-Group-Guided Strategy. Adv. Synth. Catal 2020, 362, 4513–4542;
- An X-D; Xiao J, Recent advances in hydride transfer-involved C(sp3)─H activation reactions. Org. Chem. Front 2021, 8, 1364–1383;
- Basak S; Winfrey L; Kustiana BA; Melen RL; Morrill LC; Pulis AP, Electron deficient borane-mediated hydride abstraction in amines: stoichiometric and catalytic processes. Chem. Soc. Rev 2021, 50, 3720–3737; - PubMed
- Caplin MJ; Foley DJ, Emergent synthetic methods for the modular advancement of sp3-rich fragments. Chem. Sci 2021, 12, 4646–4660; - PMC - PubMed
- Ohno S; Miyoshi M; Murai K; Arisawa M, Non-Directed β- or γ-C(sp3)─H Functionalization of Saturated Nitrogen-Containing Heterocycles. Synthesis 2021, 53, 2947–2960;
- He Y; Zheng Z; Yang J; Zhang X; Fan X, Recent advances in the functionalization of saturated cyclic amines. Org. Chem. Front 2021, 8, 4582–4606;
- Chen W; Seidel D, Condensation-Based Methods for the C─H Bond Functionalization of Amines. Synthesis 2021, 53, 3869–3908; - PMC - PubMed
- Deb ML; Saikia BS; Borpatra PJ; Baruah PK, Progress of Metal-Free Visible-Light-Driven α-C─H Functionalization of Tertiary Amines: A Decade Journey. Asian J. Org. Chem 2022, 11, e202100706.
-
- Recent examples of mechanistically diverse methods for amine C─H bond functionalization:Ohmatsu K; Suzuki R; Furukawa Y; Sato M; Ooi T, Zwitterionic 1,2,3-Triazolium Amidate as a Catalyst for Photoinduced Hydrogen-Atom Transfer Radical Alkylation. ACS Catal. 2020, 10, 2627–2632;
- Verma P; Richter JM; Chekshin N; Qiao JX; Yu J-Q, Iridium(I)-Catalyzed α-C(sp3)─H Alkylation of Saturated Azacycles. J. Am. Chem. Soc 2020, 142, 5117–5125; - PMC - PubMed
- Walker MM; Koronkiewicz B; Chen S; Houk KN; Mayer JM; Ellman JA, Highly Diastereoselective Functionalization of Piperidines by Photoredox-Catalyzed α-Amino C─H Arylation and Epimerization. J. Am. Chem. Soc 2020, 142, 8194–8202; - PMC - PubMed
- Chen L; Yang Y; Liu L; Gao Q; Xu S, Iridium-Catalyzed Enantioselective α-C(sp3)─H Borylation of Azacycles. J. Am. Chem. Soc 2020, 142, 12062–12068; - PubMed
- Xu L-P; Roque JB; Sarpong R; Musaev DG, Reactivity and Selectivity Controlling Factors in the Pd/Dialkylbiarylphosphine-Catalyzed C─C Cleavage/Cross-Coupling of an N-Fused Bicyclo α-Hydroxy-β-Lactam. J. Am. Chem. Soc 2020, 142, 21140–21152; - PubMed
- Feng K; Quevedo RE; Kohrt JT; Oderinde MS; Reilly U; White MC, Late-stage oxidative C(sp3)–H methylation. Nature 2020, 580, 621–627; - PMC - PubMed
- Sarver PJ; Bacauanu V; Schultz DM; DiRocco DA; Lam Y.-h.; Sherer EC; MacMillan DWC; The merger of decatungstate and copper catalysis to enable aliphatic C(sp3)─H trifluoromethylation. Nat. Chem 2020, 12, 459–467; - PubMed
- Oeschger R; Su B; Yu I; Ehinger C; Romero E; He S; Hartwig J, Diverse functionalization of strong alkyl C─H bonds by undirected borylation. Science 2020, 368, 736–741; - PMC - PubMed
- Short MA; Blackburn JM; Roizen JL, Modifying Positional Selectivity in C─H Functionalization Reactions with Nitrogen-Centered Radicals: Generalizable Approaches to 1,6-Hydrogen-Atom Transfer Processes. Synlett 2020, 31, 102–116; - PMC - PubMed
- Trindade AF; Faulkner EL; Leach AG; Nelson A; Marsden SP, Fragment-oriented synthesis: β-elaboration of cyclic amine fragments using enecarbamates as platform intermediates. Chem. Commun 2020, 56, 8802–8805; - PubMed
- Holmberg-Douglas N; Choi Y; Aquila B; Huynh H; Nicewicz DA, β-Functionalization of Saturated Aza-Heterocycles Enabled by Organic Photoredox Catalysis. ACS Catal. 2021, 11, 3153–3158; - PMC - PubMed
- Yi M-J; Zhang H-X; Xiao T-F; Zhang J-H; Feng Z-T; Wei L-P; Xu G-Q; Xu P-F, Photoinduced Metal-Free α-C(sp3)─H Carbamoylation of Saturated Aza-Heterocycles via Rationally Designed Organic Photocatalyst. ACS Catal. 2021, 11, 3466–3472;
- Aguilera EY; Sanford MS, Palladium-Mediated Cγ−H Functionalization of Alicyclic Amines. Angew. Chem. Int. Ed 2021, 60, 11227–11230; - PMC - PubMed
- Chang Y; Cao M; Chan JZ; Zhao C; Wang Y; Yang R; Wasa M, Enantioselective Synthesis of N-Alkylamines through β-Amino C─H Functionalization Promoted by Cooperative Actions of B(C6F5)3 and a Chiral Lewis Acid Co-Catalyst. J. Am. Chem. Soc 2021, 143, 2441–2455; - PMC - PubMed
- Yue W-J; Day CS; Martin R, Site-Selective Defluorinative sp3 C─H Alkylation of Secondary Amides. J. Am. Chem. Soc 2021, 143, 6395–6400; - PubMed
- Koperniku A; Schafer LL, Zirconium Catalyzed Hydroaminoalkylation for the Synthesis of α-Arylated Amines and N-Heterocycles. Chem. Eur. J 2021, 27, 6334–6339; - PubMed
- Novaes LFT; Ho JSK; Mao K; Liu K; Tanwar M; Neurock M; Villemure E; Terrett JA; Lin S, Exploring Electrochemical C(sp3)─H Oxidation for the Late-Stage Methylation of Complex Molecules. J. Am. Chem. Soc 2022, 144, 1187–1197; - PubMed
- Rodrigalvarez J; Reeve LA; Miró J; Gaunt MJ, Pd(II)-Catalyzed Enantioselective C(sp3)─H Arylation of Cyclopropanes and Cyclobutanes Guided by Tertiary Alkylamines. J. Am. Chem. Soc 2022, 144, 3939–3948; - PMC - PubMed
- Shu X; Zhong D; Lin Y; Qin X; Huo H, Modular Access to Chiral α-(Hetero)aryl Amines via Ni/Photoredox-Catalyzed Enantioselective Cross-Coupling. J. Am. Chem. Soc 2022, 144, 8797–8806; - PubMed
- Feng T; Wang S; Liu Y; Liu S; Qiu Y, Electrochemical Desaturative β-Acylation of Cyclic N-Aryl Amines. Angew. Chem. Int. Ed 2022, 61, e202115178; - PubMed
- Gong Y; Su L; Zhu Z; Ye Y; Gong H, Nickel-Catalyzed Thermal Redox Functionalization of C(sp3)─H Bonds with Carbon Electrophiles. Angew. Chem. Int. Ed 2022, 61, e202201662; - PubMed
- Lee W; Kim D; Seo S; Chang S, Photoinduced α-C─H Amination of Cyclic Amine Scaffolds Enabled by Polar-Radical Relay. Angew. Chem. Int. Ed 2022, 61, e202202971. - PubMed
-
- Selected reviews on the utility of α-amino nitriles:Enders D; Shilvock JP, Some recent applications of α-amino nitrile chemistry. Chem. Soc. Rev 2000, 29, 359–373;
- Fleming FF; Zhang Z, Cyclic nitriles: tactical advantages in synthesis. Tetrahedron 2005, 61, 747–789;
- Opatz T, The chemistry of deprotonated α-aminonitriles. Synthesis 2009, 1941–1959;
- Lahm G; Orejarena Pacheco JC; Opatz T, Rearrangements of Nitrile-Stabilized Ammonium Ylides. Synthesis 2014, 46, 2413–2421;
- Grundke C; Vierengel N; Opatz T, α-Aminonitriles: From Sustainable Preparation to Applications in Natural Product Synthesis. Chem. Rec 2020, 20, 989–1016. - PubMed
-
- Selected articles on oxidative amine α-cyanation of tertiary or protected amines:Andreades S; Zahnow EW, Anodic cyanations of aromatic compounds. J. Amer. Chem. Soc 1969, 91, 4181–90;
- Chiba T; Takata Y, Anodic cyanation of tertiary aliphatic and heterocyclic amines. J. Org. Chem 1977, 42, 2973–7;
- Groutas WC; Essawi M; Portoghese PS, α-Cyanation of tertiary amines. Synth. Commun 1980, 10, 495–502;
- Sundberg RJ; Theret MH; Wright L, Oxidation of amines by dichlorodicyanoquinone (DDQ) with trapping by trimethylsilyl cyanide. Org. Prep. Proced. Int 1994, 26, 386–9;
- Murahashi S-I; Komiya N; Terai H; Nakae T, Aerobic ruthenium-catalyzed oxidative cyanation of tertiary amines with sodium cyanide. J. Am. Chem. Soc 2003, 125, 15312–15313; - PubMed
- North M, Oxidative synthesis of alpha-amino nitriles from tertiary amines. Angew. Chem. Int. Ed 2004, 43, 4126–4128; - PubMed
- Li Z; Li C-J, Highly efficient CuBr-catalyzed cross-dehydrogenative coupling (CDC) between tetrahydroisoquinolines and activated methylene compounds. Eur. J. Org. Chem 2005, 3173–3176;
- Tajima T; Nakajima A, Direct oxidative cyanation based on the concept of site isolation. J. Am. Chem. Soc 2008, 130, 10496-+; - PubMed
- Han W; Ofial AR, Iron catalyzed oxidative cyanation of tertiary amines. Chem. Commun 2009, 5024–5026; - PubMed
- Shu XZ; Xia XF; Yang YF; Ji KG; Liu XY; Liang YM, Selective Functionalization of sp(3) C-H Bonds Adjacent to Nitrogen Using (Diacetoxyiodo)benzene (DIB). J. Org. Chem 2009, 74, 7464–7469; - PubMed
- Zhang Y; Peng H; Zhang M; Cheng YX; Zhu CJ, Gold-complexes catalyzed oxidative alpha-cyanation of tertiary amines. Chem. Commun 2011, 47, 2354–2356; - PubMed
- Rueping M; Zhu SQ; Koenigs RM, Visible-light photoredox catalyzed oxidative Strecker reaction. Chem. Commun 2011, 47, 12709–12711; - PubMed
- Allen JM; Lambert TH, Tropylium Ion Mediated alpha-Cyanation of Amines. J. Am. Chem. Soc 2011, 133, 1260–1262; - PubMed
- Hari DP; Koenig B, Eosin Y Catalyzed Visible Light Oxidative C-C and C-P bond Formation. Org. Lett 2011, 13, 3852–3855; - PubMed
- Kamijo S; Hoshikawa T; Inoue M, Photochemically Induced Radical Transformation of C(sp(3))-H Bonds to C(sp(3))-CN Bonds. Org. Lett 2011, 13, 5928–5931; - PubMed
- Freeman DB; Furst L; Condie AG; Stephenson CRJ, Functionally Diverse Nucleophilic Trapping of Iminium Intermediates Generated Utilizing Visible Light. Org. Lett 2012, 14, 94–97; - PMC - PubMed
- Hoshikawa T; Yoshioka S; Kamijo S; Inoue M, Photoinduced Direct Cyanation of C(sp3)─H Bonds. Synthesis 2013, 45, 874–887;
- Wakaki T; Sakai K; Enomoto T; Kondo M; Masaoka S; Oisaki K; Kanai M, C(sp3)─H Cyanation Promoted by Visible-Light Photoredox/Phosphate Hybrid Catalysis. Chem. Eur. J 2018, 24, 8051–8055; - PubMed
- Rao GA; Periasamy M, Oxidative Functionalization of Cyclic N-Arylamines with Nitromethane and TMSCN Using the T-HYDRO/t-BuOK System. Synthesis 2018, 50, 617–624;
- Mudithanapelli C; Dhorma LP; Kim M.-h., PIFA-Promoted, Solvent-Controlled Selective Functionalization of C(sp2)─H or C(sp3)─H: Nitration via C─N Bond Cleavage of CH3NO2, Cyanation, or Oxygenation in Water. Org. Lett 2019, 21, 3098–3102; - PubMed
- Xia Q; Li Y; Cheng L; Liang X; Cao C; Dai P; Deng H; Zhang W; Wang Q, Electron Donor–Acceptor Complex-Initiated Photochemical Cyanation for the Preparation of α-Amino Nitriles. Org. Lett 2020, 22, 9638–9643; - PubMed
- Kim K; Lee S; Hong SH, Direct C(sp3)─H Cyanation Enabled by a Highly Active Decatungstate Photocatalyst. Org. Lett 2021, 23, 5501–5505. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

