Topical Glaucoma Therapy Is Associated With Alterations of the Ocular Surface Microbiome
- PMID: 36036910
- PMCID: PMC9434984
- DOI: 10.1167/iovs.63.9.32
Topical Glaucoma Therapy Is Associated With Alterations of the Ocular Surface Microbiome
Abstract
Purpose: To investigate the ocular surface microbiome of patients with unilateral or asymmetric glaucoma being treated with topical ophthalmic medications in one eye and to determine whether microbial community changes were related to measures of ocular surface disease.
Methods: V3-V4 16S rRNA sequencing was conducted on ocular surface swabs collected from both eyes of 17 subjects: 10 patients with asymmetric/unilateral glaucoma using topical glaucoma therapy on only one eye and seven age-matched, healthy controls with no history of ocular disease or eyedrop use. Samples were categorized into three groups: patients' glaucomatous eye treated with eyedrops, patients' contralateral eye without eyedrops, and healthy control eyes. Comparisons were made for microbial diversity and composition, with differences in composition tested for association with ocular surface disease measures including tear meniscus height, tear break-up time, and Dry Eye Questionnaire.
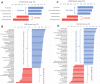
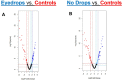
Results: Samples obtained from the patients' treated and untreated eyes both had significantly greater alpha-diversity and relative abundance of gram-negative organisms compared to healthy controls. The microbial composition of patient eyes was associated with decreased tear meniscus height and tear break-up time, whereas metagenomic predictions, based on 16S rRNA data, suggested increased synthesis of lipopolysaccharide.
Conclusions: The ocular surface microbiome of patients taking unilateral preserved glaucoma drops is characterized by a highly diverse array of gram-negative bacteria that is significantly different from the predominantly gram-positive microbes detected on healthy control eyes. These compositional differences were associated with decreased tear film measures and distinct inferred protein synthesis pathways, suggesting a potential link between microbial alterations and ocular surface inflammation.
Conflict of interest statement
Disclosure:
Figures



References
-
- Halpern DL, Grosskreutz CL.. Glaucomatous optic neuropathy: mechanisms of disease. Ophthalmol Clin North Am. 2002; 15(1): 61–68. - PubMed
-
- Dunn N, Mullee M, Perry H, Holmes C.. Association between dementia and infectious disease: evidence from a case-control study. Alzheimer Dis Assoc Dis. 2005; 19: 91–94. - PubMed
-
- Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T.. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007; 17: 341–349. - PubMed

