Administration of Anti-SARS-CoV-2 Monoclonal Antibodies After US Food and Drug Administration Deauthorization
- PMID: 36036937
- PMCID: PMC9425149
- DOI: 10.1001/jamanetworkopen.2022.28997
Administration of Anti-SARS-CoV-2 Monoclonal Antibodies After US Food and Drug Administration Deauthorization
Abstract
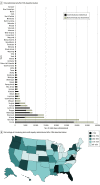
This cross-sectional study uses time-series data to evaluate the administration of bamlanivimab-etesevimab and casirivimab-imdevimab monoclonal antibody treatments for SARS-CoV-2 infection after the US Food and Drug Administration deauthorized their use in early 2022.
Conflict of interest statement
Figures

References
-
- US Food and Drug Administration . Coronavirus (COVID-19) update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the Omicron variant. January 24, 2022. Accessed March 12, 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...
-
- Levin J, Wingrove J. DeSantis touts Covid treatments after FDA says they don’t work. Bloomberg. January 25, 2022. Accessed March 12, 2022. https://www.bloomberg.com/news/articles/2022-01-25/desantis-touts-covid-...
-
- US Department of Health and Human Services . COVID-19 reported patient impact and hospital capacity by state timeseries. 2022. Accessed March 12, 2022. https://healthdata.gov/Hospital/COVID-19-Reported-Patient-Impact-and-Hos...
-
- Centers for Disease Control and Prevention . Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. 2022. Accessed March 12, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
-
- Centers for Medicare and Medicaid Services . COVID-19 vaccines and monoclonal antibodies. Accessed March 12, 2022. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/co...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

