Cytology, High-Risk Human Papillomavirus Testing and Serum CA19-9 in a Large Cohort of Patients with Invasive Cervical Adenocarcinomas: Correlation with a New Pathogenetic Classification
- PMID: 36037112
- PMCID: PMC9741895
- DOI: 10.31557/APJCP.2022.23.8.2599
Cytology, High-Risk Human Papillomavirus Testing and Serum CA19-9 in a Large Cohort of Patients with Invasive Cervical Adenocarcinomas: Correlation with a New Pathogenetic Classification
Abstract
Aims: This study aims to investigate the screening value of cytology, high-risk human papillomavirus (hrHPV) testing and serum CA19-9 in cervical adenocarcinomas.
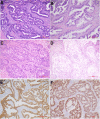
Materials and methods: We employed HPV RNA in situ hybridization and immunohistochemistry to reclassify 209 cervical adenocarcinomas according to the International Endocervical Adenocarcinoma Criteria and Classification (IECC). We analyzed the diagnostic value of cytology, hrHPV testing and serum CA19-9 in these tumors and their detection variance among IECC histotypes.
Results: We found that the sensitivity of cytology or hrHPV test alone was 74.1% (129/174) or 72% (131/182), respectively. Non-HPV related adenocarcinoma showed a lower detection rate of cytology (60%, 27/45 vs. 79.1%, 102/129, p=0.017) or hrHPV testing (9.8%, 4/41 vs. 90.1%, 127/141, p<0.0001), compared to HPV-related adenocarcinomas. The cytology and hrHPV co-testing significantly demonstrated a higher sensitivity (151/165, 91.5%) than single test alone (p<0.001). Nevertheless, the sensitivity of co-testing was substantially lower for gastric-type adenocarcinoma (GAC) (74.1%, 20/27) than that for non-GAC (94.9%, 131/138) (p=0.001). Serum CA19-9 (>40 U/mL) identified 44.1% (15/34) GACs including 75% (6/8) that were missed by co-testing, much higher than for non-GACs (10.7%, 19/177; p<0.001). The combination of cytology, hrHPV test and serum CA19-9 enhanced the detection rate of GACs (92.9%, 26/28).
Conclusion: We conclude that cytology and hrHPV co-testing is not very effective for non-HPV related adenocarcinoma, particularly for GAC. As such, additional serum CA19-9 should be given in women with potential cancer risks.
Keywords: CA19-9; Cytology; cervical adenocarcinoma; gastric-type; high-risk human papillomavirus (hrHPV) testing.
Figures
Similar articles
-
Retrospective analysis of cytology and high-risk HPV testing in 1067 endocervical adenocarcinomas and precursor lesions.Cancer Cytopathol. 2024 Jun;132(6):340-347. doi: 10.1002/cncy.22802. Epub 2024 Feb 19. Cancer Cytopathol. 2024. PMID: 38373111
-
History of high-risk HPV and Pap test results in a large cohort of patients with invasive cervical carcinoma: experience from the largest women's hospital in China.Cancer Cytopathol. 2015 Jul;123(7):421-7. doi: 10.1002/cncy.21545. Epub 2015 May 8. Cancer Cytopathol. 2015. PMID: 25955972
-
The clinical utility of extended high-risk HPV genotyping in risk-stratifying women with L-SIL cytology: A retrospective study of 8726 cases.Cancer Cytopathol. 2022 Jul;130(7):542-550. doi: 10.1002/cncy.22573. Epub 2022 Mar 21. Cancer Cytopathol. 2022. PMID: 35312217
-
Aptima HPV messenger RNA testing and histopathologic follow-up in women with HSIL cytology: A study emphasizing additional review of HPV-negative cases.Cancer Cytopathol. 2021 Aug;129(8):622-631. doi: 10.1002/cncy.22421. Epub 2021 Mar 25. Cancer Cytopathol. 2021. PMID: 33764649 Review.
-
Accuracy and effectiveness of HPV mRNA testing in cervical cancer screening: a systematic review and meta-analysis.Lancet Oncol. 2022 Jul;23(7):950-960. doi: 10.1016/S1470-2045(22)00294-7. Epub 2022 Jun 13. Lancet Oncol. 2022. PMID: 35709810
References
-
- Adams J, Sasieni P. Changing rates of adenocarcinoma and adenosquamous carcinoma of the cervix in England. Lancet. 2001;357:1490–3. - PubMed
-
- An HJ, Kim KR, Kim IS, et al. Prevalence of human papillomavirus DNA in various histological subtypes of cervical adenocarcinoma: a population-based study. Mod Pathol. 2005;18:528–34. - PubMed
-
- Bagde ND, Bagde MN, Lone ZA. Relationship between serum tumor markers, CA-125, CEA, CA19-9, LDH, and βHCG with histopathology and age in women with ovarian tumors. Asian Pac J Cancer Biol. 2020;5:167–71.
-
- Borras G, Molina R, Xercavins J, Ballesta A, Iglesias J. Tumor Antigens CA 19 9, CA 125, and CEA in carcinoma of the uterine cervix. Gynecol Oncol. 1995;57:205–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

