A protein of capillary endothelial cells, GPIHBP1, is crucial for plasma triglyceride metabolism
- PMID: 36037340
- PMCID: PMC9457329
- DOI: 10.1073/pnas.2211136119
A protein of capillary endothelial cells, GPIHBP1, is crucial for plasma triglyceride metabolism
Abstract
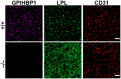
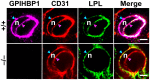
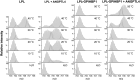
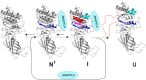
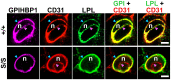
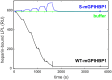
GPIHBP1, a protein of capillary endothelial cells (ECs), is a crucial partner for lipoprotein lipase (LPL) in the lipolytic processing of triglyceride-rich lipoproteins. GPIHBP1, which contains a three-fingered cysteine-rich LU (Ly6/uPAR) domain and an intrinsically disordered acidic domain (AD), captures LPL from within the interstitial spaces (where it is secreted by parenchymal cells) and shuttles it across ECs to the capillary lumen. Without GPIHBP1, LPL remains stranded within the interstitial spaces, causing severe hypertriglyceridemia (chylomicronemia). Biophysical studies revealed that GPIHBP1 stabilizes LPL structure and preserves LPL activity. That discovery was the key to crystallizing the GPIHBP1-LPL complex. The crystal structure revealed that GPIHBP1's LU domain binds, largely by hydrophobic contacts, to LPL's C-terminal lipid-binding domain and that the AD is positioned to project across and interact, by electrostatic forces, with a large basic patch spanning LPL's lipid-binding and catalytic domains. We uncovered three functions for GPIHBP1's AD. First, it accelerates the kinetics of LPL binding. Second, it preserves LPL activity by inhibiting unfolding of LPL's catalytic domain. Third, by sheathing LPL's basic patch, the AD makes it possible for LPL to move across ECs to the capillary lumen. Without the AD, GPIHBP1-bound LPL is trapped by persistent interactions between LPL and negatively charged heparan sulfate proteoglycans (HSPGs) on the abluminal surface of ECs. The AD interrupts the HSPG interactions, freeing LPL-GPIHBP1 complexes to move across ECs to the capillary lumen. GPIHBP1 is medically important; GPIHBP1 mutations cause lifelong chylomicronemia, and GPIHBP1 autoantibodies cause some acquired cases of chylomicronemia.
Keywords: endothelial cells; lipoprotein lipase; triglycerides.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Electrostatic sheathing of lipoprotein lipase is essential for its movement across capillary endothelial cells.J Clin Invest. 2022 Mar 1;132(5):e157500. doi: 10.1172/JCI157500. J Clin Invest. 2022. PMID: 35229724 Free PMC article.
-
Structure of the lipoprotein lipase-GPIHBP1 complex that mediates plasma triglyceride hydrolysis.Proc Natl Acad Sci U S A. 2019 Jan 29;116(5):1723-1732. doi: 10.1073/pnas.1817984116. Epub 2018 Dec 17. Proc Natl Acad Sci U S A. 2019. PMID: 30559189 Free PMC article.
-
A disordered acidic domain in GPIHBP1 harboring a sulfated tyrosine regulates lipoprotein lipase.Proc Natl Acad Sci U S A. 2018 Jun 26;115(26):E6020-E6029. doi: 10.1073/pnas.1806774115. Epub 2018 Jun 13. Proc Natl Acad Sci U S A. 2018. PMID: 29899144 Free PMC article.
-
GPIHBP1 and Plasma Triglyceride Metabolism.Trends Endocrinol Metab. 2016 Jul;27(7):455-469. doi: 10.1016/j.tem.2016.04.013. Epub 2016 May 14. Trends Endocrinol Metab. 2016. PMID: 27185325 Free PMC article. Review.
-
GPIHBP1 and Lipoprotein Lipase, Partners in Plasma Triglyceride Metabolism.Cell Metab. 2019 Jul 2;30(1):51-65. doi: 10.1016/j.cmet.2019.05.023. Cell Metab. 2019. PMID: 31269429 Free PMC article. Review.
Cited by
-
Dynamic metabolism of endothelial triglycerides protects against atherosclerosis in mice.J Clin Invest. 2024 Jan 4;134(4):e170453. doi: 10.1172/JCI170453. J Clin Invest. 2024. PMID: 38175710 Free PMC article.
-
Impact of Selective Peroxisome Proliferator-Activated Receptor (PPAR)-α Modulators and Fibrates on Microvascular Disease: Is There Still Room?Curr Atheroscler Rep. 2025 Mar 20;27(1):39. doi: 10.1007/s11883-025-01292-0. Curr Atheroscler Rep. 2025. PMID: 40111592 Review.
-
Mechanosensitive PIEZO2 channels shape coronary artery development.Nat Cardiovasc Res. 2025 Jul;4(7):921-937. doi: 10.1038/s44161-025-00677-3. Epub 2025 Jun 27. Nat Cardiovasc Res. 2025. PMID: 40579458 Free PMC article.
-
Aspirin impedes non-small cell lung cancer development via fine-tuning the CD36 localization regulated by GPIHBP1.Transl Lung Cancer Res. 2025 Feb 28;14(2):491-512. doi: 10.21037/tlcr-2024-1174. Epub 2025 Feb 27. Transl Lung Cancer Res. 2025. PMID: 40114952 Free PMC article.
-
Single-cell RNA sequencing of the carotid artery and femoral artery of rats exposed to hindlimb unloading.Cell Mol Life Sci. 2025 Jan 21;82(1):50. doi: 10.1007/s00018-024-05572-x. Cell Mol Life Sci. 2025. PMID: 39833543 Free PMC article.
References
-
- Korn E. D., Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J. Biol. Chem. 215, 1–14 (1955). - PubMed
-
- Korn E. D., Clearing factor, a heparin-activated lipoprotein lipase. II. Substrate specificity and activation of coconut oil. J. Biol. Chem. 215, 15–26 (1955). - PubMed
-
- Fielding C. J., Human lipoprotein lipase. I. Purification and substrate specificity. Biochim. Biophys. Acta 206, 109–117 (1970). - PubMed
-
- LaRosa J. C., Levy R. I., Herbert P., Lux S. E., Fredrickson D. S., A specific apoprotein activator for lipoprotein lipase. Biochem. Biophys. Res. Commun. 41, 57–62 (1970). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

