Breaking down antibiotic resistance in methicillin-resistant Staphylococcus aureus: Combining antimicrobial photodynamic and antibiotic treatments
- PMID: 36037346
- PMCID: PMC9457041
- DOI: 10.1073/pnas.2208378119
Breaking down antibiotic resistance in methicillin-resistant Staphylococcus aureus: Combining antimicrobial photodynamic and antibiotic treatments
Abstract
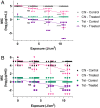
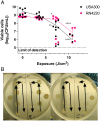
The widespread use of antibiotics drives the evolution of antimicrobial-resistant bacteria (ARB), threatening patients and healthcare professionals. Therefore, the development of novel strategies to combat resistance is recognized as a global healthcare priority. The two methods to combat ARB are development of new antibiotics or reduction in existing resistances. Development of novel antibiotics is a laborious and slow-progressing task that is no longer a safe reserve against looming risks. In this research, we suggest a method for reducing resistance to extend the efficacious lifetime of current antibiotics. Antimicrobial photodynamic therapy (aPDT) is used to generate reactive oxygen species (ROS) via the photoactivation of a photosensitizer. ROS then nonspecifically damage cellular components, leading to general impairment and cell death. Here, we test the hypothesis that concurrent treatment of bacteria with antibiotics and aPDT achieves an additive effect in the elimination of ARB. Performing aPDT with the photosensitizer methylene blue in combination with antibiotics chloramphenicol and tetracycline results in significant reductions in resistance for two methicillin-resistant Staphylococcus aureus (MRSA) strains, USA300 and RN4220. Additional resistant S. aureus strain and antibiotic combinations reveal similar results. Taken together, these results suggest that concurrent aPDT consistently decreases S. aureus resistance by improving susceptibility to antibiotic treatment. In turn, this development exhibits an alternative to overcome some of the growing MRSA challenge.
Keywords: antibiotic resistance; antimicrobial-resistant bacteria; methicillin-resistant Staphylococcus aureus; methylene blue; photodynamic therapy.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Centers for Disease Control and Prevention, Antibiotic Resistance Threats in the United States 2019 (Centers for Disease Control and Prevention, US Department of Health and Human Services, 2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

