Novel antiinflammatory biologics shaped by parasite-host coevolution
- PMID: 36037362
- PMCID: PMC9457177
- DOI: 10.1073/pnas.2202795119
Novel antiinflammatory biologics shaped by parasite-host coevolution
Abstract
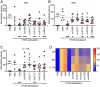
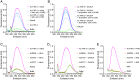
Parasitic helminth infections, while a major cause of neglected tropical disease burden, negatively correlate with the incidence of immune-mediated inflammatory diseases such as inflammatory bowel diseases (IBD). To evade expulsion, helminths have developed sophisticated mechanisms to regulate their host's immune responses. Controlled experimental human helminth infections have been assessed clinically for treating inflammatory conditions; however, such a radical therapeutic modality has challenges. An alternative approach is to harness the immunomodulatory properties within the worm's excretory-secretory (ES) complement, its secretome. Here, we report a biologics discovery and validation pipeline to generate and screen in vivo a recombinant cell-free secretome library of helminth-derived immunomodulatory proteins. We successfully expressed 78 recombinant ES proteins from gastrointestinal hookworms and screened the crude in vitro translation reactions for anti-IBD properties in a mouse model of acute colitis. After statistical filtering and ranking, 20 proteins conferred significant protection against various parameters of colitis. Lead candidates from distinct protein families, including annexins, transthyretins, nematode-specific retinol-binding proteins, and SCP/TAPS were identified. Representative proteins were produced in mammalian cells and further validated, including ex vivo suppression of inflammatory cytokine secretion by T cells from IBD patient colon biopsies. Proteins identified herein offer promise as novel, safe, and mechanistically differentiated biologics for treating the globally increasing burden of inflammatory diseases.
Keywords: IBD; helminth; hookworm; protein; therapeutic.
Conflict of interest statement
Competing interest statement: S.M.R., R.R., P.R.G., and A.L. are coinventors on a provisional patent application (AU 2021900769). A.L. and P.R.G. are founders and shareholders of Macrobiome Therapeutics, which is developing hookworm-derived proteins and drugs treating inflammatory conditions.
Figures



References
-
- Anonymous; GBD 2017 Inflammatory Bowel Disease Collaborators, The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 17–30 (2020). - PMC - PubMed
-
- Zuo T., Kamm M. A., Colombel J. F., Ng S. C., Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 15, 440–452 (2018). - PubMed
-
- Coskun M., Vermeire S., Nielsen O. H., Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol. Sci. 38, 127–142 (2017). - PubMed
-
- D’Haens G. R., Sartor R. B., Silverberg M. S., Petersson J., Rutgeerts P., Future directions in inflammatory bowel disease management. J. Crohn’s Colitis 8, 726–734 (2014). - PubMed
-
- Danese S., Vuitton L., Peyrin-Biroulet L., Biologic agents for IBD: Practical insights. Nat. Rev. Gastroenterol. Hepatol. 12, 537–545 (2015). - PubMed

