LAPTM5 mediates immature B cell apoptosis and B cell tolerance by regulating the WWP2-PTEN-AKT pathway
- PMID: 36037365
- PMCID: PMC9457450
- DOI: 10.1073/pnas.2205629119
LAPTM5 mediates immature B cell apoptosis and B cell tolerance by regulating the WWP2-PTEN-AKT pathway
Abstract
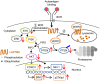
Elimination of autoreactive developing B cells is an important mechanism to prevent autoantibody production. However, how B cell receptor (BCR) signaling triggers apoptosis of immature B cells remains poorly understood. We show that BCR stimulation up-regulates the expression of the lysosomal-associated transmembrane protein 5 (LAPTM5), which in turn triggers apoptosis of immature B cells through two pathways. LAPTM5 causes BCR internalization, resulting in decreased phosphorylation of SYK and ERK. In addition, LAPTM5 targets the E3 ubiquitin ligase WWP2 for lysosomal degradation, resulting in the accumulation of its substrate PTEN. Elevated PTEN levels suppress AKT phosphorylation, leading to increased FOXO1 expression and up-regulation of the cell cycle inhibitor p27Kip1 and the proapoptotic molecule BIM. In vivo, LAPTM5 is involved in the elimination of autoreactive B cells and its deficiency exacerbates autoantibody production. Our results reveal a previously unidentified mechanism that contributes to immature B cell apoptosis and B cell tolerance.
Keywords: B cell tolerance; E3 ubiquitin ligase; apoptosis; immature B cell; lysosomal-associated transmembrane protein 5.
Conflict of interest statement
The authors declare no competing interest.
Figures

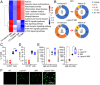
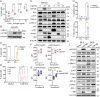
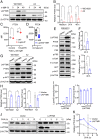

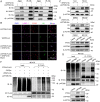
References
-
- Wang Y., Liu J., Burrows P. D., Wang J. Y., B cell development and maturation. Adv. Exp. Med. Biol. 1254, 1–22 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

