Cholesterol inhibits human voltage-gated proton channel hHv1
- PMID: 36037383
- PMCID: PMC9457259
- DOI: 10.1073/pnas.2205420119
Cholesterol inhibits human voltage-gated proton channel hHv1
Abstract
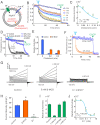
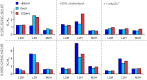
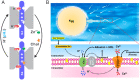
Although human sperm is morphologically mature in the epididymis, it cannot fertilize eggs before capacitation. Cholesterol efflux from the sperm plasma membrane is a key molecular event essential for cytoplasmic alkalinization and hyperactivation, but the underlying mechanism remains unclear. The human voltage-gated proton (hHv1) channel functions as an acid extruder to regulate intracellular pHs of many cell types, including sperm. Aside from voltage and pH, Hv channels are also regulated by distinct ligands, such as Zn2+ and albumin. In the present work, we identified cholesterol as an inhibitory ligand of the hHv1 channel and further investigated the underlying mechanism using the single-molecule fluorescence resonance energy transfer (smFRET) approach. Our results indicated that cholesterol inhibits the hHv1 channel by stabilizing the voltage-sensing S4 segment at resting conformations, a similar mechanism also utilized by Zn2+. Our results suggested that the S4 segment is the central gating machinery in the hHv1 channel, on which voltage and distinct ligands are converged to regulate channel function. Identification of membrane cholesterol as an inhibitory ligand provides a mechanism by which the hHv1 channel regulates fertilization by linking the cholesterol efflux with cytoplasmic alkalinization, a change that triggers calcium influx through the CatSper channel. These events finally lead to hyperactivation, a remarkable change in the mobility pattern indicating fertilization competence of human sperm.
Keywords: cholesterol; ion channel; ligand gating; structural dynamics; voltage gating.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

