Longitudinal Changes in MRI Muscle Morphometry and Composition in People With Inclusion Body Myositis
- PMID: 36038279
- PMCID: PMC10513877
- DOI: 10.1212/WNL.0000000000200776
Longitudinal Changes in MRI Muscle Morphometry and Composition in People With Inclusion Body Myositis
Abstract
Background and objectives: Limited data suggest that quantitative MRI (qMRI) measures have potential to be used as trial outcome measures in sporadic inclusion body myositis (sIBM) and as a noninvasive assessment tool to study sIBM muscle pathologic processes. Our aim was to evaluate changes in muscle structure and composition using a comprehensive multiparameter set of qMRI measures and to assess construct validity and responsiveness of qMRI measures in people with sIBM.
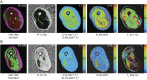
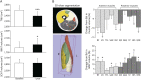
Methods: This was a prospective observational cohort study with assessments at baseline (n = 30) and 1 year (n = 26). qMRI assessments include thigh muscle volume (TMV), inter/intramuscular adipose tissue (IMAT), muscle fat fraction (FF), muscle inflammation (T2 relaxation time), IMAT from T2* relaxation (T2*-IMAT), intermuscular connective tissue from T2* relaxation (T2*-IMCT), and muscle macromolecular structure from the magnetization transfer ratio (MTR). Physical performance assessments include sIBM Physical Functioning Assessment (sIFA), 6-minute walk distance, and quantitative muscle testing of the quadriceps. Correlations were assessed using the Spearman correlation coefficient. Responsiveness was assessed using the standardized response mean (SRM).
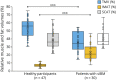
Results: After 1 year, we observed a reduction in TMV (6.8%, p < 0.001) and muscle T2 (6.7%, p = 0.035), an increase in IMAT (9.7%, p < 0.001), FF (11.2%, p = 0.030), connective tissue (22%, p = 0.995), and T2*-IMAT (24%, p < 0.001), and alteration in muscle macromolecular structure (ΔMTR = -26%, p = 0.002). A decrease in muscle T2 correlated with an increase in T2*-IMAT (r = -0.47, p = 0.008). Deposition of connective tissue and IMAT correlated with deterioration in sIFA (r = 0.38, p = 0.032; r = 0.34, p = 0.048; respectively), whereas a decrease in TMV correlated with a decrease in quantitative muscle testing (r = 0.36, p = 0.035). The most responsive qMRI measures were T2*-IMAT (SRM = 1.50), TMV (SRM = -1.23), IMAT (SRM = 1.20), MTR (SRM = -0.83), and T2 relaxation time (SRM = -0.65).
Discussion: Progressive deterioration in muscle quality measured by qMRI is associated with a decline in physical performance. Inflammation may play a role in triggering fat infiltration into muscle. qMRI provides valid and responsive measures that might prove valuable in sIBM experimental trials and assessment of muscle pathologic processes.
Classification of evidence: This study provides Class I evidence that qMRI outcome measures are associated with physical performance measures in patients with sIBM.
© 2022 American Academy of Neurology.
Conflict of interest statement
D. Laurent, R. Roubenoff, and D.A. Papanicolaou are employees of Novartis and, as such, may be eligible for Novartis stock and stock options. J. Riek is an employee of BioTel Research, which is an imaging CRO that was contracted by Novartis Pharma AG to perform the image analysis in this study. P. Houston was also an employee of Novartis at the time of the study. A. Nagy and S. Pieper are employees of Isomics Inc., which is a technology development company that was contracted by Novartis Pharma AG to perform the semiautomatic image segmentation for individual muscle volume determination. A. Nagy is also an employee of the University of Szeged, Szeged, Hungary. M.G. Hanna receives research funding from the Medical Research Council UK and has previously acted as a consultant for Novartis and for Orphazyme. P.M. Machado has received grants and/or honoraria from AbbVie, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche, and UCB Pharma. The other authors declare no relevant disclosures. Go to
Figures
Comment in
-
Quantitative MRI Biomarkers as Potential Inclusion Body Myositis Clinical Trial Endpoints.Neurology. 2022 Aug 30;99(9):361-362. doi: 10.1212/WNL.0000000000200872. Epub 2022 Jun 3. Neurology. 2022. PMID: 36038280 No abstract available.
References
-
- Greenberg SA. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol. 2019;15(5):257-272. - PubMed
-
- Jabari D, Vedanarayanan VV, Barohn RJ, Dimachkie MM. Update on inclusion body myositis. Curr Rheumatol Rep. 2018;20(8):52. - PubMed
-
- Schmidt J, Dalakas MC. Inclusion body myositis: from immunopathology and degenerative mechanisms to treatment perspectives. Expert Rev Clin Immunol. 2013;9(11):1125-1133. - PubMed
-
- Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear cells in myopathies. III: immunoelectron microscopy aspects of cell-mediated muscle fiber injury. Ann Neurol. 1986;19(2):112-125. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
