Targeting proliferative retinopathy: Arginase 1 limits vitreoretinal neovascularization and promotes angiogenic repair
- PMID: 36038541
- PMCID: PMC9424300
- DOI: 10.1038/s41419-022-05196-8
Targeting proliferative retinopathy: Arginase 1 limits vitreoretinal neovascularization and promotes angiogenic repair
Abstract
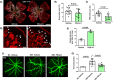
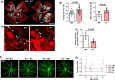
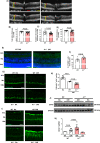
Current therapies for treatment of proliferative retinopathy focus on retinal neovascularization (RNV) during advanced disease and can trigger adverse side-effects. Here, we have tested a new strategy for limiting neurovascular injury and promoting repair during early-stage disease. We have recently shown that treatment with a stable, pegylated drug form of the ureohydrolase enzyme arginase 1 (A1) provides neuroprotection in acute models of ischemia/reperfusion injury, optic nerve crush, and ischemic stroke. Now, we have determined the effects of this treatment on RNV, vascular repair, and retinal function in the mouse oxygen-induced retinopathy (OIR) model of retinopathy of prematurity (ROP). Our studies in the OIR model show that treatment with pegylated A1 (PEG-A1), inhibits pathological RNV, promotes angiogenic repair, and improves retinal function by a mechanism involving decreased expression of TNF, iNOS, and VEGF and increased expression of FGF2 and A1. We further show that A1 is expressed in myeloid cells and areas of RNV in retinal sections from mice with OIR and human diabetic retinopathy (DR) patients and in blood samples from ROP patients. Moreover, studies using knockout mice with hemizygous deletion of A1 show worsened RNV and retinal injury, supporting the protective role of A1 in limiting the OIR-induced pathology. Collectively, A1 is critically involved in reparative angiogenesis and neuroprotection in OIR. Pegylated A1 may offer a novel therapy for limiting retinal injury and promoting repair during proliferative retinopathy.
© 2022. The Author(s).
Conflict of interest statement
AF, RBC, and RWC have a pending patent on the use of arginase 1 as a treatment for ischemic retinopathies. PN-MC is the chief executive officer of Bio-Cancer Treatment International Limited and holds stocks or shares in Bio-Cancer Treatment International Limited.
Figures




References
-
- Nuzzi R, Tridico F. Local and systemic complications after intravitreal administration of anti-vascular endothelial growth factor agents in the treatment of different ocular diseases: a five-year retrospective study. Semin Ophthalmol. 2015;30:129–35. doi: 10.3109/08820538.2013.835833. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

