Revealing nanostructures in brain tissue via protein decrowding by iterative expansion microscopy
- PMID: 36038771
- PMCID: PMC9551354
- DOI: 10.1038/s41551-022-00912-3
Revealing nanostructures in brain tissue via protein decrowding by iterative expansion microscopy
Abstract
Many crowded biomolecular structures in cells and tissues are inaccessible to labelling antibodies. To understand how proteins within these structures are arranged with nanoscale precision therefore requires that these structures be decrowded before labelling. Here we show that an iterative variant of expansion microscopy (the permeation of cells and tissues by a swellable hydrogel followed by isotropic hydrogel expansion, to allow for enhanced imaging resolution with ordinary microscopes) enables the imaging of nanostructures in expanded yet otherwise intact tissues at a resolution of about 20 nm. The method, which we named 'expansion revealing' and validated with DNA-probe-based super-resolution microscopy, involves gel-anchoring reagents and the embedding, expansion and re-embedding of the sample in homogeneous swellable hydrogels. Expansion revealing enabled us to use confocal microscopy to image the alignment of pre-synaptic calcium channels with post-synaptic scaffolding proteins in intact brain circuits, and to uncover periodic amyloid nanoclusters containing ion-channel proteins in brain tissue from a mouse model of Alzheimer's disease. Expansion revealing will enable the further discovery of previously unseen nanostructures within cells and tissues.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Figures



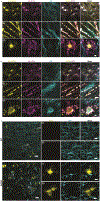
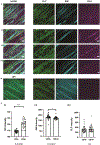
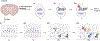


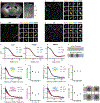
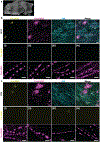
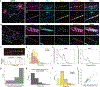
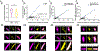
Comment in
-
Visualizing protein nanostructures in intact brain.Nat Biotechnol. 2022 Oct;40(10):1432. doi: 10.1038/s41587-022-01511-y. Nat Biotechnol. 2022. PMID: 36207600 No abstract available.
References
-
- Sydor AM, Czymmek KJ, Puchner EM & Mennella V Super-Resolution Microscopy: From Single Molecules to Supramolecular Assemblies. Trends in Cell Biology vol. 25 730–748 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 GM126277/GM/NIGMS NIH HHS/United States
- RM1 HG008525/HG/NHGRI NIH HHS/United States
- R56 AG069192/AG/NIA NIH HHS/United States
- R01 EB024261/EB/NIBIB NIH HHS/United States
- DP1 GM133052/GM/NIGMS NIH HHS/United States
- RF1 MH124606/MH/NIMH NIH HHS/United States
- R01 MH110932/MH/NIMH NIH HHS/United States
- R00 GM126277/GM/NIGMS NIH HHS/United States
- R37 MH080046/MH/NIMH NIH HHS/United States
- R01 MH119826/MH/NIMH NIH HHS/United States
- R01 AG070831/AG/NIA NIH HHS/United States
- U24 NS109113/NS/NINDS NIH HHS/United States
- R01 NS102730/NS/NINDS NIH HHS/United States
- RF1 AG054321/AG/NIA NIH HHS/United States
- DP1 NS087724/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

