Intranasal Xylitol for the Treatment of COVID-19 in the Outpatient Setting: A Pilot Study
- PMID: 36039203
- PMCID: PMC9395150
- DOI: 10.7759/cureus.27182
Intranasal Xylitol for the Treatment of COVID-19 in the Outpatient Setting: A Pilot Study
Abstract
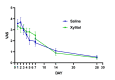
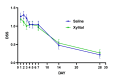
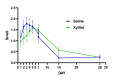
It is well known that acute COVID-19 infection can present with a variety of symptoms, including fever, cough, rhinitis, loss of taste, and the cardinal sign of loss of smell (anosmia). Recently, nasal irrigations with saline and other agents have shown promise for the treatment of COVID-19. Xylitol has been shown to display virucidal effects against SARS-CoV-2. This study aimed to examine the efficacy of xylitol as an adjunct treatment for COVID-19 in an outpatient setting. In a randomized controlled double-blinded fashion, a total of 50 participants (F=30) consented to participate in this study. It was a population of 18 to 65 years of age, with polymerase chain reaction confirmed for SARS-COV-2 by nasopharyngeal swab, less than three days from the start of symptoms. This study's primary endpoint was time to clinical recovery, defined as the change from baseline to end of treatment in COVID-19 symptoms. Outcome variables were the changes in visual analog scale (VAS) and daily symptoms score (DSS) on Days 1-7, 14, and 28 after the initiation of the 14-day treatment. There were no differences between the treatment groups in any demographic and subject characteristics-related variables, including vaccination status. None of the patients were hospitalized, or required emergency visits in addition to no adverse reactions were reported. There were no statistically significant interactions found for VAS (P=0.124), DSS (P=0.448), and sense of smell (P=0.667). The proportion of patients reporting nasal congestion was higher (X2=5.05; P=0.025) in the xylitol (XYL) group (73.1%) vs. the saline (SAL) group (41.7%) on Day 4, and on Day 7 (X2=5.72; P=0.017) XYL group (50.0%) vs. SAL group (17.4%). During Day 28 a total of two patients in the SAL group had anosmia vs. no patients with anosmia in the XYL group, although this difference did not reach statistical significance (X2=5.72; P=0.133). Results demonstrate that both xylitol and saline were equally effective in decreasing the time of symptom resolution and preventing hospitalizations, yet, persistent anosmia was only seen in the SAL group. Intranasal xylitol might play a pivotal role in preventing persistent olfactory abnormalities in post-COVID-19 patients.
Keywords: anosmia; covid-19; intranasal therapies; nasal hygiene; saline; sars-cov-2; xylitol.
Copyright © 2022, Soler et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- World Health Organization. WHO Coronavirus (COVID-19) dashboard. [ Jul; 2022 ];https://covid19.who.int/ 2022
LinkOut - more resources
Full Text Sources
Miscellaneous