HMGB1 increases RAGE expression in vascular smooth muscle cells via ERK and p-38 MAPK-dependent pathways
- PMID: 36039739
- PMCID: PMC9437367
- DOI: 10.4196/kjpp.2022.26.5.389
HMGB1 increases RAGE expression in vascular smooth muscle cells via ERK and p-38 MAPK-dependent pathways
Abstract
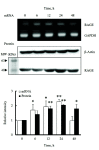
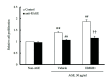
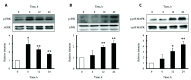
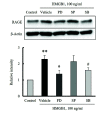
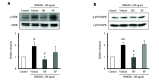
The increased expression of receptors for advanced glycation end-product (RAGE) is known as a key player in the progression of vascular remodeling. However, the precise signal pathways regulating RAGE expression in vascular smooth muscle cells (VSMCs) in the injured vasculatures are unclear. Given the importance of mitogen-activated protein kinase (MAPK) signaling in cell proliferation, we investigated the importance of MAPK signaling in high-mobility group box 1 (HMGB1)-induced RAGE expression in VSMCs. In HMGB1 (100 ng/ml)-stimulated human VSMCs, the expression of RAGE mRNA and protein was increased in association with an increase in AGE-induced VSMC proliferation. The HMGB1-induced RAGE expression was attenuated in cells pretreated with inhibitors for ERK (PD98059, 10 μM) and p38 MAPK (SB203580, 10 μM) as well as in cells deficient in ERK and p38 MAPK using siRNAs, but not in cells deficient of JNK signaling. In cells stimulated with HMGB1, the phosphorylation of ERK, JNK, and p38 MAPK was increased. This increase in ERK and p38 MAPK phosphorylation was inhibited by p38 MAPK and ERK inhibitors, respectively, but not by JNK inhibitor. Moreover, AGE-induced VSMC proliferation in HMGB1-stimulated cells was attenuated in cells treated with ERK and p38 MAPK inhibitors. Taken together, our results indicate that ERK and p38 MAPK signaling are involved in RAGE expression in HMGB1-stimulated VSMCs. Thus, the ERK/p38 MAPK-RAGE signaling axis in VSMCs was suggested as a potential therapeutic target for vascular remodeling in the injured vasculatures.
Keywords: HMGB1; MAPK; RAGE; Vascular remodeling; Vascular smooth muscle cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Hreggvidsdottir HS, Ostberg T, Wähämaa H, Schierbeck H, Aveberger AC, Klevenvall L, Palmblad K, Ottosson L, Andersson U, Harris HE. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–662. doi: 10.1189/jlb.0908548. - DOI - PubMed
-
- Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, Ilyinskaya O, Tararak E, Bobik A. Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol. 2004;24:2320–2325. doi: 10.1161/01.ATV.0000145573.36113.8a. - DOI - PubMed
-
- Cai Y, Sukhova GK, Wong HK, Xu A, Tergaonkar V, Vanhoutte PM, Tang EH. Rap1 induces cytokine production in pro-inflammatory macrophages through NFκB signaling and is highly expressed in human atherosclerotic lesions. Cell Cycle. 2015;14:3580–3592. doi: 10.1080/15384101.2015.1100771. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

