Structural insights into the dual activities of the two-barrel RNA polymerase QDE-1
- PMID: 36039765
- PMCID: PMC9508822
- DOI: 10.1093/nar/gkac727
Structural insights into the dual activities of the two-barrel RNA polymerase QDE-1
Abstract
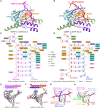
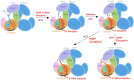
Neurospora crassa protein QDE-1, a member of the two-barrel polymerase superfamily, possesses both DNA- and RNA-dependent RNA polymerase (DdRP and RdRP) activities. The dual activities are essential for the production of double-stranded RNAs (dsRNAs), the precursors of small interfering RNAs (siRNAs) in N. crassa. Here, we report five complex structures of N-terminal truncated QDE-1 (QDE-1ΔN), representing four different reaction states: DNA/RNA-templated elongation, the de novo initiation of RNA synthesis, the first step of nucleotide condensation during de novo initiation and initial NTP loading. The template strand is aligned by a bridge-helix and double-psi beta-barrels 2 (DPBB2), the RNA product is held by DPBB1 and the slab domain. The DNA template unpairs with the RNA product at position -7, but the RNA template remains paired. The NTP analog coordinates with cations and is precisely positioned at the addition site by a rigid trigger loop and a proline-containing loop in the active center. The unique C-terminal tail from the QDE-1 dimer partner inserts into the substrate-binding cleft and plays regulatory roles in RNA synthesis. Collectively, this work elucidates the conserved mechanisms for DNA/RNA-dependent dual activities by QDE-1 and other two-barrel polymerase superfamily members.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






References
-
- Cogoni C., Macino G.. Gene silencing in Neurospora crassa requires a protein homologous to RNA-dependent RNA polymerase. Nature. 1999; 399:166–169. - PubMed
-
- Dalmay T., Hamilton A., Rudd S., Angell S., Baulcombe D.C.. An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell. 2000; 101:543–553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

