Serodiagnosis and therapeutic monitoring of New-World tegumentary leishmaniasis using synthetic type-2 glycoinositolphospholipid-based neoglycoproteins
- PMID: 36039908
- PMCID: PMC9518598
- DOI: 10.1080/22221751.2022.2114852
Serodiagnosis and therapeutic monitoring of New-World tegumentary leishmaniasis using synthetic type-2 glycoinositolphospholipid-based neoglycoproteins
Abstract
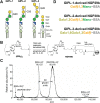
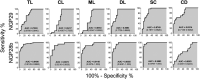
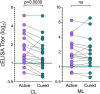
American tegumentary leishmaniasis (TL) caused by Leishmania braziliensis is characterized by a spectrum of clinical presentations, ranging from localized cutaneous ulcers (CL), mucosal (ML), or disseminated (DL) disease, to a subclinical (SC) asymptomatic form. Current diagnosis based on parasite culture and/or microscopy lacks sensitivity and specificity. Previous studies showed that patients with CL and ML have very high levels of Leishmania-specific anti-α-Gal antibodies. However, the native parasite α-Gal glycotope(s) is(are) still elusive, thus they have not yet been explored for a more accurate TL diagnosis. Using a chemiluminescent immunoassay, we evaluated the seroreactivity of TL patients across its clinical spectrum, and of endemic (EC) and nonendemic healthy controls (NEC) against three synthetic neoglycoproteins (NGP29b, NGP30b, and NGP28b), respectively comprising the L. major-derived type-2 glycoinositolphospholipid (GIPL)-1 (Galfβ1,3Manα), GIPL-2 (Galα1,3Galfβ1,3Manα), and GIPL-3 (Galα1,6Galα1,3Galfβ) glycotopes. Contrary to NGP29b and NGP30b, NGP28b exhibited high sensitivity and specificity to a CL serum pool. More importantly, NGP28b reacted strongly and specifically with individual sera from distinct clinical forms of TL, especially with SC sera, with 94% sensitivity and 97% specificity, by post-two-graph receiver-operating characteristic curve analysis. Contrary to NGP29b, NGP28b showed low cross-reactivity with Chagas disease and control (NEC/EC) sera. Additionally, seroreactivity of CL patients against NGP28b was significantly decreased after successful chemotherapy, indicating that L. braziliensis-specific anti-α-Gal antibodies may serve as an early biomarker of cure in CL. Our data also points towards the applicability of L. major type-2 GIPL-3-derived Galα1,6Galα1,3Galfβ glycotope for the serological diagnosis of American TL, particularly of the subclinical form.
Keywords: Leishmania braziliensis; anti-α-Gal antibodies; diagnostic and prognostic biomarkers; tegumentary leishmaniasis; α-Gal neoglycoproteins.
Figures


References
-
- Goto H, Lindoso JA.. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther. 2010;8(4):419–433. - PubMed
-
- Ramírez JL, Guevara P.. Persistent infections by Leishmania (Viannia) braziliensis. Mem Inst Oswaldo Cruz. 1997;92(3):333–338. - PubMed
-
- Amato VS, Tuon FF, Bacha HA, et al. . Mucosal leishmaniasis. Current scenario and prospects for treatment. Acta Trop. 2008;105:1–9. - PubMed
-
- Gaze ST, Dutra WO, Lessa M, et al. . Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol. 2006;63(1):70–78. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
