A Core Outcome Measurement Set for Pediatric Critical Care
- PMID: 36040097
- PMCID: PMC9633391
- DOI: 10.1097/PCC.0000000000003055
A Core Outcome Measurement Set for Pediatric Critical Care
Abstract
Objectives: To identify a PICU Core Outcome Measurement Set (PICU COMS), a set of measures that can be used to evaluate the PICU Core Outcome Set (PICU COS) domains in PICU patients and their families.
Design: A modified Delphi consensus process.
Setting: Four webinars attended by PICU physicians and nurses, pediatric surgeons, rehabilitation physicians, and scientists with expertise in PICU clinical care or research ( n = 35). Attendees were from eight countries and convened from the Pediatric Acute Lung Injury and Sepsis Investigators Pediatric Outcomes STudies after PICU Investigators and the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network PICU COS Investigators.
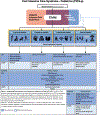
Subjects: Measures to assess outcome domains of the PICU COS are as follows: cognitive, emotional, overall (including health-related quality of life), physical, and family health. Measures evaluating social health were also considered.
Interventions: None.
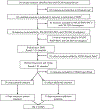
Measurements and main results: Measures were classified as general or additional based on generalizability across PICU populations, feasibility, and relevance to specific COS domains. Measures with high consensus, defined as 80% agreement for inclusion, were selected for the PICU COMS. Among 140 candidate measures, 24 were delineated as general (broadly applicable) and, of these, 10 achieved consensus for inclusion in the COMS (7 patient-oriented and 3 family-oriented). Six of the seven patient measures were applicable to the broadest range of patients, diagnoses, and developmental abilities. All were validated in pediatric populations and have normative pediatric data. Twenty additional measures focusing on specific populations or in-depth evaluation of a COS subdomain also met consensus for inclusion as COMS additional measures.
Conclusions: The PICU COMS delineates measures to evaluate domains in the PICU COS and facilitates comparability across future research studies to characterize PICU survivorship and enable interventional studies to target long-term outcomes after critical illness.
Copyright © 2022 by the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies.
Conflict of interest statement
Dr. Maddux’s institution received funding from the National Institute of Child Health and Human Development (NICHD) (K23HD096018) and the Francis Family Foundation, Parker B Francis Fellowship. Drs. Maddux, Jarvis, Killien, Meert, Olson, and Fink received support for article research from the National Institutes of Health (NIH). Drs. Jarvis, Michelson, Beers, Meert, and Fink’s institutions received funding from the NIH. Dr. Jarvis received funding from the NIH (T32 HD040686). Dr. Killien and Olson’s institutions received funding from the NICHD. Dr. Choong’s institution received funding from the AFP Innovation Fund; she received funding from McMaster University. Dr. Michelson’s institution received funding from The National Palliative Care Research Center, and the Greenwall Foundation. Dr. Lee’s institution received funding from the National Medical Research Council, Singapore. Dr. Slomine received funding from the National Academy of Neuropsychology and Cambridge University Press. Dr. Beers’ institution received funding from the National Football League Brain Health Study. Dr. Morrow received funding from EduPro, Imperial College Press, the University of Cape Town, and the South African Society of Physiotherapy. Dr. Fink’s institution received funding from the Neurocritical Care Society; she received funding from the Child Nervous Society and the American Board of Pediatrics CCM Subsection member. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
Comment in
-
Measure to Improve Like PROs: Patient-Related Outcomes in Survivors of Pediatric Critical Illness.Pediatr Crit Care Med. 2022 Nov 1;23(11):946-949. doi: 10.1097/PCC.0000000000003084. Epub 2022 Nov 3. Pediatr Crit Care Med. 2022. PMID: 36326460 No abstract available.
References
-
- Pinto NP, Rhinesmith EW, Kim TY, Ladner PH, Pollack MM: Long-Term Function After Pediatric Critical Illness: Results From the Survivor Outcomes Study. Pediatr Crit Care Med 2017; 18:e122–e130. - PubMed
-
- Choong K, Fraser D, Al-Harbi S et al. : Functional Recovery in Critically Ill Children, the “WeeCover” Multicenter Study. Pediatr Crit Care Med 2018; 19:145–154. - PubMed
-
- Namachivayam P, Shann F, Shekerdemian L et al. : Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med 2010; 11:549–555. - PubMed
-
- Ong C, Lee JH, Leow MK, Puthucheary ZA: Functional Outcomes and Physical Impairments in Pediatric Critical Care Survivors: A Scoping Review. Pediatr Crit Care Med 2016; 17:e247–259. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

