Molecular and Kinetic Characterization of MOX-9, a Plasmid-Mediated Enzyme Representative of a Novel Sublineage of MOX-Type Class C β-Lactamases
- PMID: 36040170
- PMCID: PMC9487596
- DOI: 10.1128/aac.00595-22
Molecular and Kinetic Characterization of MOX-9, a Plasmid-Mediated Enzyme Representative of a Novel Sublineage of MOX-Type Class C β-Lactamases
Abstract
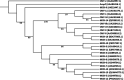
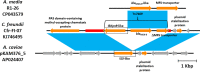
The MOX lineage of β-lactamases includes a group of molecular class C enzymes (AmpCs) encoded by genes mobilized from the chromosomes of Aeromonas spp. to plasmids. MOX-9, previously identified as a plasmid-encoded enzyme from a Citrobacter freundii isolate, belongs to a novel sublineage of MOX enzymes, derived from the resident Aeromonas media AmpC. The blaMOX-9 gene was found to be carried on a transposon, named Tn7469, likely responsible for its mobilization to plasmidic context. MOX-9 was overexpressed in Escherichia coli, purified, and subjected to biochemical characterization. Kinetic analysis showed a relatively narrow-spectrum profile with strong preference for cephalosporin substrates, with some differences compared with MOX-1 and MOX-2. MOX-9 was not inhibited by clavulanate and sulbactam, while both tazobactam and avibactam acted as inhibitors in the micromolar range.
Keywords: MOX; enzyme kineticss; β-lactamases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Perilli M, Felici A, Oratore A, Cornaglia G, Bonfiglio G, Rossolini GM, Amicosante G. 1996. Characterization of the chromosomal cephalosporinases produced by Acinetobacter lwoffii and Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother 40:715–719. 10.1128/AAC.40.3.715. - DOI - PMC - PubMed
-
- Tavio MM, Perilli M, Vila J, Becerro P, Casanas L, Amicosante G, Jimenez de Anta MT. 2004. Salicylate decreases production of AmpC type β-lactamases and increases susceptibility to β-lactams in a Morganella morganii clinical isolate. FEMS Microbiol Lett 238:139–144. 10.1016/j.femsle.2004.07.030. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

