The gateway to guanine nucleotides: Allosteric regulation of IMP dehydrogenases
- PMID: 36040265
- PMCID: PMC9375230
- DOI: 10.1002/pro.4399
The gateway to guanine nucleotides: Allosteric regulation of IMP dehydrogenases
Abstract
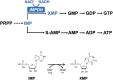
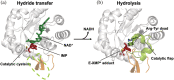
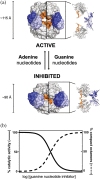
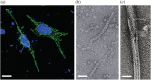
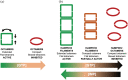
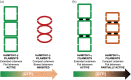
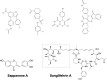
Inosine 5'-monophosphate dehydrogenase (IMPDH) is an evolutionarily conserved enzyme that mediates the first committed step in de novo guanine nucleotide biosynthetic pathway. It is an essential enzyme in purine nucleotide biosynthesis that modulates the metabolic flux at the branch point between adenine and guanine nucleotides. IMPDH plays key roles in cell homeostasis, proliferation, and the immune response, and is the cellular target of several drugs that are widely used for antiviral and immunosuppressive chemotherapy. IMPDH enzyme is tightly regulated at multiple levels, from transcriptional control to allosteric modulation, enzyme filamentation, and posttranslational modifications. Herein, we review recent developments in our understanding of the mechanisms of IMPDH regulation, including all layers of allosteric control that fine-tune the enzyme activity.
Keywords: IMP dehydrogenase; allosteric regulation; enzyme filamentation; protein structure and function; purine nucleotide biosynthesis.
© 2022 The Authors. Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Figures






Similar articles
-
Diversity of mechanisms to control bacterial GTP homeostasis by the mutually exclusive binding of adenine and guanine nucleotides to IMP dehydrogenase.Protein Sci. 2022 May;31(5):e4314. doi: 10.1002/pro.4314. Protein Sci. 2022. PMID: 35481629 Free PMC article.
-
Induction of IMPDH-Based Cytoophidia by a Probable IMP-Dependent ARL13B-IMPDH Interaction.Biochemistry (Mosc). 2024 Dec;89(12):2287-2291. doi: 10.1134/S0006297924120162. Biochemistry (Mosc). 2024. PMID: 39865040
-
A Nucleotide-Dependent Conformational Switch Controls the Polymerization of Human IMP Dehydrogenases to Modulate their Catalytic Activity.J Mol Biol. 2019 Mar 1;431(5):956-969. doi: 10.1016/j.jmb.2019.01.020. Epub 2019 Jan 18. J Mol Biol. 2019. PMID: 30664871
-
IMPDH dysregulation in disease: a mini review.Biochem Soc Trans. 2022 Feb 28;50(1):71-82. doi: 10.1042/BST20210446. Biochem Soc Trans. 2022. PMID: 35191957 Free PMC article. Review.
-
[The determination and clinical application of inosine 5’-monophosphate dehydrogenase activity].Yao Xue Xue Bao. 2016 Nov;51(11):1666-73. Yao Xue Xue Bao. 2016. PMID: 29908108 Review. Chinese.
Cited by
-
Deciphering the allosteric regulation of mycobacterial inosine-5'-monophosphate dehydrogenase.Nat Commun. 2024 Aug 6;15(1):6673. doi: 10.1038/s41467-024-50933-6. Nat Commun. 2024. PMID: 39107302 Free PMC article.
-
Insight into the role of the Bateman domain at the molecular and physiological levels through engineered IMP dehydrogenases.Protein Sci. 2023 Aug;32(8):e4703. doi: 10.1002/pro.4703. Protein Sci. 2023. PMID: 37338125 Free PMC article.
-
An IMPDH2 variant associated with neurodevelopmental disorder disrupts purine biosynthesis and somitogenesis.bioRxiv [Preprint]. 2025 May 11:2025.05.09.652712. doi: 10.1101/2025.05.09.652712. bioRxiv. 2025. PMID: 40654686 Free PMC article. Preprint.
-
Anandamide Modulates Thermal Avoidance in Caenorhabditis elegans Through Vanilloid and Cannabinoid Receptor Interplay.Neurochem Res. 2024 Sep;49(9):2423-2439. doi: 10.1007/s11064-024-04186-w. Epub 2024 Jun 7. Neurochem Res. 2024. PMID: 38847909
-
Effect of urea and squaramide IMPDH inhibitors on C. parvum: in vitro trial design impacts the assessment of drug efficacy.Int J Parasitol Drugs Drug Resist. 2025 Aug;28:100592. doi: 10.1016/j.ijpddr.2025.100592. Epub 2025 Apr 15. Int J Parasitol Drugs Drug Resist. 2025. PMID: 40319744 Free PMC article.
References
-
- Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. - PubMed
-
- Jayaram H, Cooney DA, Grusch M, Krupitza G. Consequences of IMP dehydrogenase inhibition, and its relationship to cancer and apoptosis. Curr Med Chem. 1999;6:561–574. - PubMed
-
- Braun‐Sand SB, Peetz M. Inosine monophosphate dehydrogenase as a target for antiviral, anticancer, antimicrobial and immunosuppressive therapeutics. Future Med Chem. 2009;2:81–92. - PubMed
-
- Cuny GD, Suebsuwong C, Ray SS. Inosine‐5′‐monophosphate dehydrogenase (IMPDH) inhibitors: A patent and scientific literature review (2002‐2016). Expert Opin Ther Pat. 2017;27:677–690. - PubMed
-
- Nagano N, Orengo CA, Thornton JM. One fold with many functions: The evolutionary relationships between TIM barrel families based on their sequences, structures and functions. J Mol Biol. 2002;321:741–765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

