Neuroscout, a unified platform for generalizable and reproducible fMRI research
- PMID: 36040302
- PMCID: PMC9489206
- DOI: 10.7554/eLife.79277
Neuroscout, a unified platform for generalizable and reproducible fMRI research
Abstract
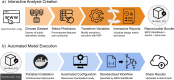
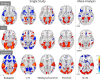
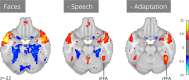
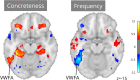
Functional magnetic resonance imaging (fMRI) has revolutionized cognitive neuroscience, but methodological barriers limit the generalizability of findings from the lab to the real world. Here, we present Neuroscout, an end-to-end platform for analysis of naturalistic fMRI data designed to facilitate the adoption of robust and generalizable research practices. Neuroscout leverages state-of-the-art machine learning models to automatically annotate stimuli from dozens of fMRI studies using naturalistic stimuli-such as movies and narratives-allowing researchers to easily test neuroscientific hypotheses across multiple ecologically-valid datasets. In addition, Neuroscout builds on a robust ecosystem of open tools and standards to provide an easy-to-use analysis builder and a fully automated execution engine that reduce the burden of reproducible research. Through a series of meta-analytic case studies, we validate the automatic feature extraction approach and demonstrate its potential to support more robust fMRI research. Owing to its ease of use and a high degree of automation, Neuroscout makes it possible to overcome modeling challenges commonly arising in naturalistic analysis and to easily scale analyses within and across datasets, democratizing generalizable fMRI research.
Keywords: fMRI; generalizability; human; naturalistic; neuroinformatics; neuroscience; open source; reproducibility.
© 2022, de la Vega, Rocca et al.
Conflict of interest statement
Ad, RR, RB, CM, JM, JK, PH, SG, RP, TY No competing interests declared
Figures


References
-
- Abadi M, Agarwal A, Barham P, Brevdo E, Chen Z, Citro C, Corrado GS, Davis A, Dean J, Devin M, Ghemawat S, Goodfellow I, Harp A, Irving G, Isard M, Jia Y, Jozefowicz R, Kaiser L, Kudlur M, Levenberg J, Mané D, Monga R, Moore S, Murray D, Olah C, Schuster M, Shlens J, Steiner B, Sutskever I, Talwar K, Tucker P, Vanhoucke V, Vasudevan V, Viégas F, Vinyals O, Warden P, Wattenberg M, Wicke M, Yu Y, Zheng X. TensorFlow: Large-Scale Machine Learning on Heterogeneous Systems. arXiv. 2015 https://arxiv.org/abs/1603.04467
-
- Alejandro de la V. Neuroscout. swh:1:rev:ed79e9cf4b1ee1320a2d43c72e95f3fd3619c9b7Software Heritage. 2022a https://archive.softwareheritage.org/swh:1:dir:a358fe02406a82b5e06c79e8c...
-
- Alejandro de la V. PyNS. swh:1:rev:db62c4b7eb4d1f23ff5f4e59617a58c4206e68eaSoftware Heritage. 2022b https://archive.softwareheritage.org/swh:1:dir:9e0df08165a466b72ab1a93b4...
-
- Alejandro de la V. Neuroscout. swh:1:rev:d4399ce98ec44f20dcd1072454d0bd57c9c14ac2Software Heritage. 2022c https://archive.softwareheritage.org/swh:1:dir:39e35f7067bafa4e1a7ac0fa2...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

