Primary Multiparametric Quantitative Brain MRI: State-of-the-Art Relaxometric and Proton Density Mapping Techniques
- PMID: 36040334
- PMCID: PMC9524578
- DOI: 10.1148/radiol.211519
Primary Multiparametric Quantitative Brain MRI: State-of-the-Art Relaxometric and Proton Density Mapping Techniques
Abstract
This review on brain multiparametric quantitative MRI (MP-qMRI) focuses on the primary subset of quantitative MRI (qMRI) parameters that represent the mobile ("free") and bound ("motion-restricted") proton pools. Such primary parameters are the proton densities, relaxation times, and magnetization transfer parameters. Diffusion qMRI is also included because of its wide implementation in complete clinical MP-qMRI application. MP-qMRI advances were reviewed over the past 2 decades, with substantial progress observed toward accelerating image acquisition and increasing mapping accuracy. Areas that need further investigation and refinement are identified as follows: (a) the biologic underpinnings of qMRI parameter values and their changes with age and/or disease and (b) the theoretical limitations implicitly built into most qMRI mapping algorithms that do not distinguish between the different spatial scales of voxels versus spin packets, the central physical object of the Bloch theory. With rapidly improving image processing techniques and continuous advances in computer hardware, MP-qMRI has the potential for implementation in a wide range of clinical applications. Currently, three emerging MP-qMRI applications are synthetic MRI, macrostructural qMRI, and microstructural tissue modeling.
© RSNA, 2022.
Conflict of interest statement
Figures


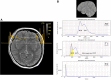
![Voxel types according to spatial scalability. Three different voxel types
with increasing inner complexity from left to right (simulation colors: blue =
cerebrospinal fluid [CSF], red = gray matter [GM], green = white matter [WM]).
Type 1, which contains pure CSF (left), is fully scalable, meaning that the
quantitative MRI (qMRI) parameters at the spin-packet and voxel scales are
equal. Type 2 voxels contain a typical structured soft tissue (eg, WM or GM),
leading to a distribution of correlation times and magnetization transfer
effects, as reviewed in the text. Type 3 voxels are difficult for qMRI
algorithms because of partial volume effects.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/803e/9524578/19c6cbe6806e/radiol.211519.fig7.gif)



References
-
- Cho S , Jones D , Reddick WE , Ogg RJ , Steen RG . Establishing norms for age-related changes in proton T1 of human brain tissue in vivo . Magn Reson Imaging 1997. ; 15 ( 10 ): 1133 – 1143 . - PubMed
-
- Suzuki S , Sakai O , Jara H . Combined volumetric T1, T2 and secular-T2 quantitative MRI of the brain: age-related global changes (preliminary results) . Magn Reson Imaging 2006. ; 24 ( 7 ): 877 – 887 . - PubMed
-
- Saito N , Sakai O , Ozonoff A , Jara H . Relaxo-volumetric multispectral quantitative magnetic resonance imaging of the brain over the human lifespan: global and regional aging patterns . Magn Reson Imaging 2009. ; 27 ( 7 ): 895 – 906 . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

