Hypoxia and low temperature upregulate transferrin to induce hypercoagulability at high altitude
- PMID: 36040436
- PMCID: PMC10653030
- DOI: 10.1182/blood.2022016410
Hypoxia and low temperature upregulate transferrin to induce hypercoagulability at high altitude
Abstract
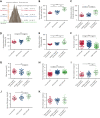
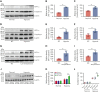
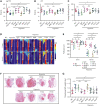
Studies have shown significantly increased thromboembolic events at high altitude. We recently reported that transferrin could potentiate blood coagulation, but the underlying mechanism for high altitude-related thromboembolism is still poorly understood. Here, we examined the activity and concentration of plasma coagulation factors and transferrin in plasma collected from long-term human residents and short-stay mice exposed to varying altitudes. We found that the activities of thrombin and factor XIIa (FXIIa) along with the concentrations of transferrin were significantly increased in the plasma of humans and mice at high altitudes. Furthermore, both hypoxia (6% O2) and low temperature (0°C), 2 critical high-altitude factors, enhanced hypoxia-inducible factor 1α (HIF-1α) levels to promote the expression of the transferrin gene, whose enhancer region contains HIF-1α binding site, and consequently, to induce hypercoagulability by potentiating thrombin and FXIIa. Importantly, thromboembolic disorders and pathological insults in mouse models induced by both hypoxia and low temperature were ameliorated by transferrin interferences, including transferrin antibody treatment, transferrin downregulation, and the administration of our designed peptides that inhibit the potentiation of transferrin on thrombin and FXIIa. Thus, low temperature and hypoxia upregulated transferrin expression-promoted hypercoagulability. Our data suggest that targeting the transferrin-coagulation pathway is a novel and potentially powerful strategy against thromboembolic events caused by harmful environmental factors under high-altitude conditions.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




Comment in
-
Transferrin-induced thrombosis in hypoxia.Blood. 2022 Nov 10;140(19):2006-2008. doi: 10.1182/blood.2022018181. Blood. 2022. PMID: 36355465 No abstract available.
References
-
- Bendz B, Rostrup M, Sevre K, Andersen TO, Sandset PM. Association between acute hypobaric hypoxia and activation of coagulation in human beings. Lancet. 2000;356(9242):1657–1658. - PubMed
-
- Monge C, León-Velarde F. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol Rev. 1991;71(4):1135–1172. - PubMed
-
- Gassmann M, Muckenthaler MU. Adaptation of iron requirement to hypoxic conditions at high altitude. J Appl Physiol. 2015;119(12):1432–1440. - PubMed
-
- Robach P, Cairo G, Gelfi C, et al. Strong iron demand during hypoxia-induced erythropoiesis is associated with down-regulation of iron-related proteins and myoglobin in human skeletal muscle. Blood. 2007;109(11):4724–4731. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

