A "multi-omics" analysis of blood-brain barrier and synaptic dysfunction in APOE4 mice
- PMID: 36040482
- PMCID: PMC9435921
- DOI: 10.1084/jem.20221137
A "multi-omics" analysis of blood-brain barrier and synaptic dysfunction in APOE4 mice
Abstract
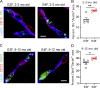
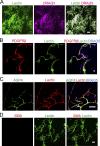
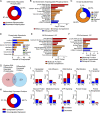
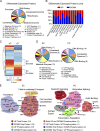
Apolipoprotein E4 (APOE4), the main susceptibility gene for Alzheimer's disease, leads to blood-brain barrier (BBB) breakdown in humans and mice. Remarkably, BBB dysfunction predicts cognitive decline and precedes synaptic deficits in APOE4 human carriers. How APOE4 affects BBB and synaptic function at a molecular level, however, remains elusive. Using single-nucleus RNA-sequencing and phosphoproteome and proteome analysis, we show that APOE4 compared with APOE3 leads to an early disruption of the BBB transcriptome in 2-3-mo-old APOE4 knock-in mice, followed by dysregulation in protein signaling networks controlling cell junctions, cytoskeleton, clathrin-mediated transport, and translation in brain endothelium, as well as transcription and RNA splicing suggestive of DNA damage in pericytes. Changes in BBB signaling mechanisms paralleled an early, progressive BBB breakdown and loss of pericytes, which preceded postsynaptic interactome disruption and behavioral deficits that developed 2-5 mo later. Thus, dysregulated signaling mechanisms in endothelium and pericytes in APOE4 mice reflect a molecular signature of a progressive BBB failure preceding changes in synaptic function and behavior.
© 2022 Barisano et al.
Conflict of interest statement
Disclosures: J.K. Ichida reported personal fees from Acurastem, Modulo Bio, and Biomarin Pharmaceutical and “other” from Spinogenix and Vesalius Therapeutics outside the submitted work. No other disclosures were reported.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

