Normalization of cerebral hemodynamics after hematopoietic stem cell transplant in children with sickle cell disease
- PMID: 36040484
- PMCID: PMC9936296
- DOI: 10.1182/blood.2022016618
Normalization of cerebral hemodynamics after hematopoietic stem cell transplant in children with sickle cell disease
Abstract
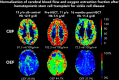
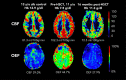
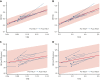
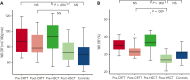
Children with sickle cell disease (SCD) demonstrate cerebral hemodynamic stress and are at high risk of strokes. We hypothesized that curative hematopoietic stem cell transplant (HSCT) normalizes cerebral hemodynamics in children with SCD compared with pre-transplant baseline. Whole-brain cerebral blood flow (CBF) and oxygen extraction fraction (OEF) were measured by magnetic resonance imaging 1 to 3 months before and 12 to 24 months after HSCT in 10 children with SCD. Three children had prior overt strokes, 5 children had prior silent strokes, and 1 child had abnormal transcranial Doppler ultrasound velocities. CBF and OEF of HSCT recipients were compared with non-SCD control participants and with SCD participants receiving chronic red blood cell transfusion therapy (CRTT) before and after a scheduled transfusion. Seven participants received matched sibling donor HSCT, and 3 participants received 8 out of 8 matched unrelated donor HSCT. All received reduced-intensity preparation and maintained engraftment, free of hemolytic anemia and SCD symptoms. Pre-transplant, CBF (93.5 mL/100 g/min) and OEF (36.8%) were elevated compared with non-SCD control participants, declining significantly 1 to 2 years after HSCT (CBF, 72.7 mL/100 g per minute; P = .004; OEF, 27.0%; P = .002), with post-HSCT CBF and OEF similar to non-SCD control participants. Furthermore, HSCT recipients demonstrated greater reduction in CBF (-19.4 mL/100 g/min) and OEF (-8.1%) after HSCT than children with SCD receiving CRTT after a scheduled transfusion (CBF, -0.9 mL/100 g/min; P = .024; OEF, -3.3%; P = .001). Curative HSCT normalizes whole-brain hemodynamics in children with SCD. This restoration of cerebral oxygen reserve may explain stroke protection after HSCT in this high-risk patient population.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.L.H. has spouse employed at Pfizer Inc, is a consultant for bluebird bio and for Global Blood Therapeutics, and has received research funding from Global Blood Therapeutics and Forma Therapeutics. M.E.F. owns equity at Proclara Biociences, participated in a scientific advisory board with compensation at bluebird bio, and is a consultant for Global Blood Therapeutics. S.S. is a consultant for Aruvant Sciences and participated in scientific advisory boards with compensation at Bristol Myers Squibb, Graphite Bio, and Janssen Pharmaceuticals. M.B.B. is employed at Open Cell Technologies Inc. The remaining authors declare no competing financial interests.
Figures
Comment in
-
Anemia and brain hypoxia.Blood. 2023 Jan 26;141(4):327-328. doi: 10.1182/blood.2022018229. Blood. 2023. PMID: 36701169 Free PMC article. No abstract available.
References
-
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. - PubMed
-
- Kinney TR, Sleeper LA, Wang WC, et al. Silent cerebral infarcts in sickle cell anemia: a risk factor analysis. The Cooperative Study of Sickle Cell Disease. Pediatrics. 1999;103(3):640–645. - PubMed
-
- Bernaudin F, Verlhac S, Arnaud C, et al. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 2015;125(10):1653–1661. - PubMed
-
- Scothorn DJ, Price C, Schwartz D, et al. Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J Pediatr. 2002;140(3):48–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

