Borate Buffer as a Key Player in Cu-Based Homogeneous Electrocatalytic Water Oxidation
- PMID: 36040755
- PMCID: PMC9828671
- DOI: 10.1002/chem.202202407
Borate Buffer as a Key Player in Cu-Based Homogeneous Electrocatalytic Water Oxidation
Abstract
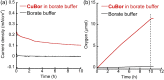
Borate buffer was found to have both structural and functional roles within a low-cost tri-copper electrocatalyst for homogeneous water oxidation that exhibits a high turnover frequency of 310 s-1 . The borate buffer was shown to facilitate the catalytic activity by both bridging the three Cu ions and participating in O-O bond formation. Phosphate and acetate buffers did not show such roles, making borate a unique player in this catalytic system.
Keywords: borate buffer; copper; electrocatalysis; trinuclear; water oxidation.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

