Activation of targetable inflammatory immune signaling is seen in myelodysplastic syndromes with SF3B1 mutations
- PMID: 36040792
- PMCID: PMC9427103
- DOI: 10.7554/eLife.78136
Activation of targetable inflammatory immune signaling is seen in myelodysplastic syndromes with SF3B1 mutations
Abstract
Background: Mutations in the SF3B1 splicing factor are commonly seen in myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), yet the specific oncogenic pathways activated by mis-splicing have not been fully elucidated. Inflammatory immune pathways have been shown to play roles in the pathogenesis of MDS, though the exact mechanisms of their activation in splicing mutant cases are not well understood.
Methods: RNA-seq data from SF3B1 mutant samples was analyzed and functional roles of interleukin-1 receptor-associated kinase 4 (IRAK4) isoforms were determined. Efficacy of IRAK4 inhibition was evaluated in preclinical models of MDS/AML.
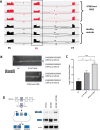
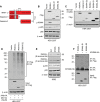
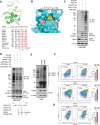
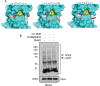
Results: RNA-seq splicing analysis of SF3B1 mutant MDS samples revealed retention of full-length exon 6 of IRAK4, a critical downstream mediator that links the Myddosome to inflammatory NF-kB activation. Exon 6 retention leads to a longer isoform, encoding a protein (IRAK4-long) that contains the entire death domain and kinase domain, leading to maximal activation of NF-kB. Cells with wild-type SF3B1 contain smaller IRAK4 isoforms that are targeted for proteasomal degradation. Expression of IRAK4-long in SF3B1 mutant cells induces TRAF6 activation leading to K63-linked ubiquitination of CDK2, associated with a block in hematopoietic differentiation. Inhibition of IRAK4 with CA-4948, leads to reduction in NF-kB activation, inflammatory cytokine production, enhanced myeloid differentiation in vitro and reduced leukemic growth in xenograft models.
Conclusions: SF3B1 mutation leads to expression of a therapeutically targetable, longer, oncogenic IRAK4 isoform in AML/MDS models.
Funding: This work was supported by Cincinnati Children's Hospital Research Foundation, Leukemia Lymphoma Society, and National Institute of Health (R35HL135787, RO1HL111103, RO1DK102759, RO1HL114582), Gabrielle's Angel Foundation for Cancer Research, and Edward P. Evans Foundation grants to DTS. AV is supported by Edward P. Evans Foundation, National Institute of Health (R01HL150832, R01HL139487, R01CA275007), Leukemia and Lymphoma Society, Curis and a gift from the Jane and Myles P. Dempsey family. AP and JB are supported by Blood Cancer UK (grants 13042 and 19004). GC is supported by a training grant from NYSTEM. We acknowledge support of this research from The Einstein Training Program in Stem Cell Research from the Empire State Stem Cell Fund through New York State Department of Health Contract C34874GG. MS is supported by a National Institute of Health Research Training and Career Development Grant (F31HL132420).
Keywords: AML; CA4948; Emavusertib; IRAK4; MDS; SF3B1; human; medicine.
Plain language summary
Genes contain blocks of code that tell cells how to make each part of a protein. Between these blocks are sections of linking DNA, which cells remove when they are preparing to use their genes. Scientists call this process 'splicing'. Cells can splice some genes in more than one way, allowing them to make different proteins from the same genetic code. Mutations that affect the splicing process can change the way cells make their proteins, leading to disease. For example, the myelodysplastic syndromes are a group of blood cancers often caused by mutations in splicing proteins, such as SF3B1. The disorder stops blood cells from maturing and causes abnormal inflammation. So far, the link between splicing, blood cell immaturity, inflammation and cancer is not clear. To find out more, Choudhary, Pellagatti et al. looked at the spliced genetic code from people with myelodysplastic syndromes. Mutations in the splicing protein SF3B1 changed the way cells spliced an important signalling molecule known as IRAK4. Affected cells cut out less genetic code and made a longer version of this signalling protein, named IRAK4-Long. This altered protein activated inflammation and stopped blood cells from maturing. Blocking IRAK4-Long reversed the effects. It also reduced tumour formation in mice carrying affected human cells. The molecule used to block IRAK4, CA-4948 – also known as Emavusertib – is currently being evaluated in clinical trials for myelodysplastic syndromes and other types of blood cancer. The work of Choudhary, Pellagatti et al. could help scientists to design genetic tests to predict which patients might benefit from this treatment.
© 2022, Choudhary, Pellagatti et al.
Conflict of interest statement
GC, AP, BA, TB, SG, SS, SP, NS, SA, RA, SA, LS, VS, MC, KP, TB, MP, BW, AW, AS, EG, JB No competing interests declared, MS MS owns stock options in Poseida Therapeutics. The author has no other competing interests to declare, RB RB is a former employee of Curis, Inc, and holds patents patents related to CA-4948 (WO-2019089580-A1). RB also owns stocks in Curis, Inc. The author has no other competing interests to declare, MR MR is an employee of Aurigene Inc, MS MS owns stock options in Curis, Inc as an employee. The author has no other competing interests to declare, RB RKB is a named inventor on patent applications related to treating cancers with SF3B1 mutations filed by Fred Hutchinson Cancer Research Center (METHODS AND COMPOSITIONS COMPRISING BRD9 ACTIVATING THERAPIES FOR TREATING CANCERS AND RELATED DISORDERS - PCT/US2020/039645; SYNTHETIC INTRONS FOR TARGETED GENE EXPRESSION - PCT/US21/56273).The author has no other competing interests to declare, RM REM is an employee of Curis, Inc and has received honoria payments and also holds stocks in the company. The author has no other competing interests to declare, US US held grants from Bayer Healthcare and Aileron Therapauetics, and received consultancy fees from Aileron Therapeutics, Stelexis Therapeutics, Pieris Pharmaceuticals and Trillium Therapeutics. US is Director at Stelexis Therapeutics and holds stocks in the company. The author has no other competing interests to declare, DS DS received consultancy fees from Kurome Therapeutics, Treeline Biosciences, Tolero Therapeutics, and Captor Therapeutics. DS also owns stocks in Kurome Therapeutics. The author has no other competing interests to declare, AV AV has received research funding from GlaxoSmithKline, BMS, Jannsen, Incyte, MedPacto, Celgene, Novartis, Curis, Prelude and Eli Lilly and Company, has received compensation as a scientific advisor to Novartis, Stelexis Therapeutics, Acceleron Pharma, and Celgene, and has equity ownership in Throws Exception and Stelexis Therapeutics
Figures




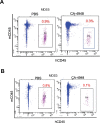



References
-
- Barreyro L, Will B, Bartholdy B, Zhou L, Todorova TI, Stanley RF, Ben-Neriah S, Montagna C, Parekh S, Pellagatti A, Boultwood J, Paietta E, Ketterling RP, Cripe L, Fernandez HF, Greenberg PL, Tallman MS, Steidl C, Mitsiades CS, Verma A, Steidl U. Overexpression of IL-1 receptor accessory protein in stem and progenitor cells and outcome correlation in AML and MDS. Blood. 2012;120:1290–1298. doi: 10.1182/blood-2012-01-404699. - DOI - PMC - PubMed
-
- Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu J, Fortenbery N, Epling-Burnette P, Van Bijnen S, Dolstra H, Cannon J, Youn J, Donatelli SS, Qin D, De Witte T, Tao J, Wang H, Cheng P, Gabrilovich DI, List A, Wei S. Induction of myelodysplasia by myeloid-derived suppressor cells. The Journal of Clinical Investigation. 2013;123:4595–4611. doi: 10.1172/JCI67580. - DOI - PMC - PubMed
-
- Dimicoli S, Wei Y, Bueso-Ramos C, Yang H, Dinardo C, Jia Y, Zheng H, Fang Z, Nguyen M, Pierce S, Chen R, Wang H, Wu C, Garcia-Manero G. Overexpression of the toll-like receptor (TLR) signaling adaptor MYD88, but lack of genetic mutation, in myelodysplastic syndromes. PLOS ONE. 2013;8:e71120. doi: 10.1371/journal.pone.0071120. - DOI - PMC - PubMed
-
- Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, Metzeler KH, Poeppl A, Ling V, Beyene J, Canty AJ, Danska JS, Bohlander SK, Buske C, Minden MD, Golub TR, Jurisica I, Ebert BL, Dick JE. Stem cell gene expression programs influence clinical outcome in human leukemia. Nature Medicine. 2011;17:1086–1093. doi: 10.1038/nm.2415. - DOI - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

