Solubility affects IL-1β-producing activity of the synthetic candidalysin peptide
- PMID: 36040970
- PMCID: PMC9426886
- DOI: 10.1371/journal.pone.0273663
Solubility affects IL-1β-producing activity of the synthetic candidalysin peptide
Abstract
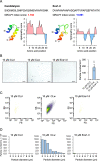
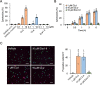
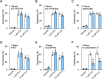
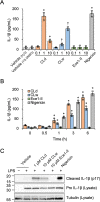
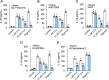
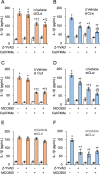
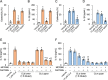
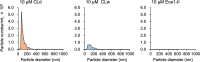
Candidalysin, a peptide toxin produced specifically from hyphae of Candida albicans, plays a crucial role in C. albicans pathogenesis in the oral cavity and vagina. Synthetic peptides have been widely used in previous studies to investigate the bioactivity of candidalysin. Although the solubility of the peptide, which is expected to have a hydrophobic property, has not been well characterized, candidalysin solutions are usually prepared in water. In this study, we prepared the synthetic peptide candidalysin in water (CLw) or in dimethyl sulfoxide (CLd) and compared their cytotoxicity and interleukin (IL)-1β-producing activity to determine whether the activity of the peptide would be affected. In addition, we evaluated whether the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome pathway or other pathways were involved in their activities. Unexpectedly, we found that CLw was not completely solubilized and contained abundant insoluble microparticles. CLw was active at comparably high concentrations (≥ 10 μM). In contrast, CLd is completely solubilized and sufficiently active at low concentrations, that is, 1 μM or less. CLw showed weak cytotoxicity and NLRP3-dependent and cathepsin B-dependent IL-1β-producing activity, whereas CLd showed strong cytotoxicity and cathepsin B-dependent IL-1β-producing activity. Fractionation of CLw revealed that NLRP3-dependent activity was caused by insoluble microparticles. Furthermore, nanoparticle tracking of CLd revealed that the peptide was present as nanoparticles with a size of 96 nm. CLw contained a small amount of such nanoparticles. Thus, the bioactivities of the synthetic peptide candidalysin, especially the IL-1β-producing activity, are affected by the solubility of the peptide depending on the solvent employed. The NLRP3-dependent activity of the synthetic peptide is caused by insoluble microparticles and may not be the intrinsic activity of candidalysin.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

