Optimization and characterization of alkaliphilic lipase from a novel Bacillus cereus NC7401 strain isolated from diesel fuel polluted soil
- PMID: 36040973
- PMCID: PMC9426928
- DOI: 10.1371/journal.pone.0273368
Optimization and characterization of alkaliphilic lipase from a novel Bacillus cereus NC7401 strain isolated from diesel fuel polluted soil
Abstract
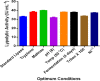
Five Bacillus cereus strains including B. cereus AVP12, B. cereus NC7401, B. cereus BDBCO1, B. cereus JF70 and B. specie JL47 isolated from the diesel fuel polluted soil adhered to the roots of Tagetes minuta were screened for lipase production with phenol red agar method. B. cereus NC7401 strain successfully expressing and secreting lipase with maximal lipolytic activity was subjected to a submerged fermentation process with five different carbon (starch, glucose, maltose, fructose, and lactose) and five different nitrogen (tryptone, ammonium nitrate, peptone, urea, yeast extract) sources to produce lipase enzyme. Maximum enzyme activity was found with starch (30.6 UmL-1), maltose (40 UmL-1), and tryptone (38.6 UmL-1), and the lipases produced using these sources were named lipase A, B, and C respectively. The total protein content of 8.56, 8.86, and 2.75 μg mL-1 were obtained from B. cereus NC7401 cultured using starch, maltose, and tryptone respectively. Lipase was stable between temperature range 30-80°C and pH 5-10 whereas optimally active at 55°C and pH 8.0. The enzyme was relatively stable for 10 days at 4°C and its optimum reaction time with the substrate was 30 minutes. It was tolerant to 1.5% (v/v) methanol as an organic solvent, 1.5% (v/v) Triton X-100 as a media additive and 1.5% (w/v) Ni2+ as a metal ion. SDS, n-hexane, and Ag+ inhibited lipolytic activity. Oil stains were removed from cotton fabric which showed oil removal efficiency enhancement in the presence of a lipase. Fat hydrolysis of 20, 24, and 30% was achieved following 6 hours of incubation of the fat particles with lipase A, B, and C respectively at a concentration of 20 mg mL-1. To as best of our knowledge, this study on lipases extracted from bacteria of Azad Kashmir, Pakistan origin has never been reported before.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Selvam K, Govarthanan M, Senbagam D, Kamala-Kannan S, Senthilkumar B, Selvankumar T. Activity and stability of bacterial cellulase immobilized on magnetic nanoparticles. Cuihua Xuebao/Chinese J Catal. 2016; 37(11):1891–1898.
-
- Al-maqtari QA, Mahdi AA. Microbial enzymes produced by fermentation and their applications in the food industry-A review. Int J of Agriculture Innov Res. 2019; 8(1):62–82.
-
- Ambasana L, Pandhi N. Optimization of process parameters for enhanced thermostable lipase production by bacillus subtilis shvsc04. Int J Adv Res. 2019; 7(11):211–221.
-
- Bhosale HJ, Uzma SZ, Bismile PC. Optimization of lipase production by thermo-alkalophilic Bacillus sp. 8C. Res J Microbiol. 2015; 10(11):523–531.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

